Weekly epirubicin plus docetaxel as first-line treatment in metastatic breast cancer
- PMID: 17940499
- PMCID: PMC2360453
- DOI: 10.1038/sj.bjc.6603982
Weekly epirubicin plus docetaxel as first-line treatment in metastatic breast cancer
Abstract
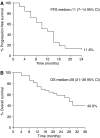
This study was designed to evaluate the efficacy and tolerability of a weekly schedule of epirubicin in combination with docetaxel in the first-line treatment of patients with metastatic breast cancer (MBC). A total of 43 women with MBC not previously treated with chemotherapy for metastatic disease received weekly epirubicin 25 mg m(-2) and docetaxel 25 mg m(-2) for a maximum of five cycles (total cumulative epirubicin dose of < or =900 mg m(-2)). Dose reduction was not permitted. Objective response and evaluation of toxicity profile were the primary study end points; time to progression and overall survival were secondary end points. Patients were followed for a median of 21 (4-38) months. Analysis was by intent to treat; 33 patients completed five cycles of therapy, and the median dose of epirubicin administered to the 43 patients was 23 mg m(-2). Twenty-five patients (58%) achieved a partial response and one (2%) achieved a complete response. An additional 12 patients (28%) had stable disease. The median time to progression was 11 months (95% confidence intervals (CI) 7-14) overall, and 13 months (95% CI 12-14) in the 26 patients who responded to treatment. Median overall survival was 25 months for responders and 14 months for nonresponders. Grade 3/4 neutropenia occurred in 16% of patients and in 6% of cycles. One patient developed cardiac toxicity (20% reduction in left ventricular ejection fraction). The combination of epirubicin plus docetaxel is highly active in MBC, with a manageable toxicity profile. Such a weekly schedule might provide a valuable treatment option for MBC.
Figures
References
-
- A’Hern RP (2001) Sample size tables for exact single-stage phase II designs. Stat Med 20: 859–866 - PubMed
-
- Bastholt L, Dalmark M, Gjedde SB, Pfeiffer P, Pendersen D, Sandeberg E, Kjaer M, Mouridsen HT, Rose C, Nielsen OS, Jakobsen P, Bentzen SM (1996) Dose–response relationship of epirubicin in the treatment of postmenopausal patients with metastatic breast cancer: a randomized study of epirubicin at four different dose levels performed by the Danish Breast Cancer Cooperative Group. J Clin Oncol 14: 1146–1155 - PubMed
-
- Brufman G, Colajori E, Ghilezan N, Lassus M, Martoni M, Perevodchikova N, Tosello C, Viaro D, Zielinski C, The Epirubicin High Dose (HEPI) Study Group (1997) Doubling epirubicin dose intensity (100 mg/m2 versus 50 mg/m2) in the FEC regimen significantly increases response rates. An international randomised phase III study in metastatic breast cancer. The Epirubicin High Dose (HEPI 010) Study Group. Ann Oncol 8: 155–162 - PubMed
-
- Burstein HJ, Manola J, Younger J, Parker LM, Craig D, Bonnell CA, Scheib R, Matulonis UA, Garber JE, Clarke KD, Shulman LN, Winer EP (2000) Docetaxel administered on a weekly basis for metastatic breast cancer. J Clin Oncol 18: 1212–1219 - PubMed
-
- Fabi A, Papaldo P, Pino MS, Ferretti G, Carlini P, Pacetti U, Di Cosimo S, Nardoni C, Giannarelli D, Sacchi I, Cognetti F (2004) Epirubicin plus docetaxel in metastatic breast cancer: escalating dose does not improve efficacy. A phase II study. Anticancer Res 24: 1963–1967 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical