The Domino Way to Heterocycles
- PMID: 17940591
- PMCID: PMC2031872
- DOI: 10.1016/j.tet.2007.03.158
The Domino Way to Heterocycles
Abstract
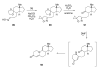
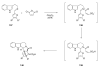
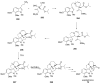
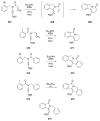
Sequential transformations enable the facile synthesis of complex target molecules from simple building blocks in a single preparative step. Their value is amplified if they also create multiple stereogenic centers. In the ongoing search for new domino processes, emphasis is usually placed on sequential reactions which occur cleanly and without forming by-products. As a prerequisite for an ideally proceeding one-pot sequential transformation, the reactivity pattern of all participating components has to be such that each building block gets involved in a reaction only when it is supposed to do so. The development of sequences that combine transformations of fundamentally different mechanisms broadens the scope of such procedures in synthetic chemistry. This mini review contains a representative sampling from the last 15 years on the kinds of reactions that have been sequenced into cascades to produce heterocyclic molecules.
Figures






























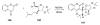





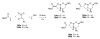




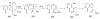









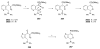










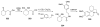


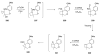




















References
-
- Katritzky A, Rees CW, Scriven EF, editors. Comprehensive Heterocyclic Chemistry. Elsevier Science; Oxford, U.K.: 1996.
-
- Kuehne ME, Matsko TH, Bohnert JC, Motyka L, Oliver-Smith D. J Org Chem. 1981;46:2002.
- Kuehne ME, Earley WG. Tetrahedron. 1983;39:3707.
- Kuehne ME, Brook CS, Xu F, Parsons R. Pure Appl Chem. 1994;66:2095.
- Nkiliza J, Vercauteren J. Tetrahedron Lett. 1991;32:1787.
- Rawal VH, Michoud C, Monestel RF. J Am Chem Soc. 1993;46:3030.
-
- Overman LE, Sworin M, Burk RM. J Org Chem. 1983;48:2685.
- Overman LE, Sugai S. Helv Chim Acta. 1985;68:745.
- Overman LE, Robertson G, Robichaud AJ. J Org Chem. 1989;54:1236.
-
- Gallagher T, Magnus P, Huffman JC. J Am Chem Soc. 1983;105:4750.
- Magnus P, Gallagher T, Brown P, Pappalardo P. Acc Chem Res. 1984;17:35.
-
- Rigby JH, Qabar MH. J Org Chem. 1993;58:4473.
- Rigby JH, Qabar M, Ahmed G, Hughes RC. Tetrahedron. 1993;49:10219.
- Rigby JH, Hughes RC, Heeg MJ. J Am Chem Soc. 1995;117:7834.
- Rigby JH, Mateo ME. Tetrahedron. 1996;52:10569.
- Rigby JH, Laurent S, Cavezza A, Heeg MJ. J Org Chem. 1998;63:5587.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
