Systems analysis of chaperone networks in the malarial parasite Plasmodium falciparum
- PMID: 17941702
- PMCID: PMC1976336
- DOI: 10.1371/journal.pcbi.0030168
Systems analysis of chaperone networks in the malarial parasite Plasmodium falciparum
Abstract
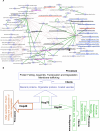
Molecular chaperones participate in the maintenance of cellular protein homeostasis, cell growth and differentiation, signal transduction, and development. Although a vast body of information is available regarding individual chaperones, few studies have attempted a systems level analysis of chaperone function. In this paper, we have constructed a chaperone interaction network for the malarial parasite, Plasmodium falciparum. P. falciparum is responsible for several million deaths every year, and understanding the biology of the parasite is a top priority. The parasite regularly experiences heat shock as part of its life cycle, and chaperones have often been implicated in parasite survival and growth. To better understand the participation of chaperones in cellular processes, we created a parasite chaperone network by combining experimental interactome data with in silico analysis. We used interolog mapping to predict protein-protein interactions for parasite chaperones based on the interactions of corresponding human chaperones. This data was then combined with information derived from existing high-throughput yeast two-hybrid assays. Analysis of the network reveals the broad range of functions regulated by chaperones. The network predicts involvement of chaperones in chromatin remodeling, protein trafficking, and cytoadherence. Importantly, it allows us to make predictions regarding the functions of hypothetical proteins based on their interactions. It allows us to make specific predictions about Hsp70-Hsp40 interactions in the parasite and assign functions to members of the Hsp90 and Hsp100 families. Analysis of the network provides a rational basis for the anti-malarial activity of geldanamycin, a well-known Hsp90 inhibitor. Finally, analysis of the network provides a theoretical basis for further experiments designed toward understanding the involvement of this important class of molecules in parasite biology.
Conflict of interest statement
Figures

References
-
- Smith DF, Whitesell L, Katsanis E. Molecular chaperones: Biology and prospects for pharmacological intervention. Pharmacol Rev. 1998;50:493–514. - PubMed
-
- Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. - PubMed
-
- Sato N, Torigoe T. The molecular chaperones in cell cycle control. Ann N Y Acad Sci. 1998;851:61–66. - PubMed
-
- Pratt WB. The Hsp90 based chaperone system: Involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med. 1998;214:420–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

