Assessing tumor progression factors by somatic gene transfer into a mouse model: Bcl-xL promotes islet tumor cell invasion
- PMID: 17941720
- PMCID: PMC2020504
- DOI: 10.1371/journal.pbio.0050276
Assessing tumor progression factors by somatic gene transfer into a mouse model: Bcl-xL promotes islet tumor cell invasion
Abstract
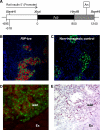
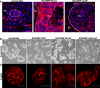
Tumors develop through multiple stages, implicating multiple effectors, but the tools to assess how candidate genes contribute to stepwise tumor progression have been limited. We have developed a novel system in which progression of phenotypes in a mouse model of pancreatic islet cell tumorigenesis can be used to measure the effects of genes introduced by cell-type-specific infection with retroviral vectors. In this system, bitransgenic mice, in which the rat insulin promoter (RIP) drives expression of both the SV40 T antigen (RIP-Tag) and the receptor for subgroup A avian leukosis virus (RIP-tva), are infected with avian viral vectors carrying cDNAs encoding candidate progression factors. Like RIP-Tag mice, RIP-Tag; RIP-tva bitransgenic mice develop isolated carcinomas by approximately 14 wk of age, after progression through well-defined stages that are similar to aspects of human tumor progression, including hyperplasia, angiogenesis, adenoma, and invasive carcinoma. When avian retroviral vectors carrying a green fluorescent protein marker were introduced into RIP-Tag; RIP-tva mice by intra-cardiac injection at the hyperplastic or early dysplastic stage of tumorigenesis, approximately 20% of the TVA-positive cells were infected and expressed green fluorescent proteins as measured by flow cytometry. Similar infection with vectors carrying cDNA encoding either of two progression factors, a dominant-negative version of cadherin 1 (dnE-cad) or Bcl-xL, accelerated the formation of islet tumors with invasive properties and pancreatic lymph node metastasis. To begin studying the mechanism by which Bcl-xL, an anti-apoptotic protein, promotes invasion and metastasis, RIP-Tag; RIP-tva pancreatic islet tumor cells were infected in vitro with RCASBP-Bcl-xL. Although no changes were observed in rates of proliferation or apoptosis, Bcl-xL altered cell morphology, remodeled the actin cytoskeleton, and down-regulated cadherin 1; it also induced cell migration and invasion, as evaluated using two-chamber transwell assays. In addition, myosin Va was identified as a novel Bcl-xL-interacting protein that might mediate the effects of Bcl-xL on tumor cell migration and invasion.
Conflict of interest statement
Figures







References
-
- Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–141. - PubMed
-
- Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, et al. Molecular classification of cancer: Class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. - PubMed
-
- Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Jonkers J, Berns A. Conditional mouse models of sporadic cancer. Nat Rev Cancer. 2002;2:251–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

