Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants
- PMID: 17941721
- PMCID: PMC2020497
- DOI: 10.1371/journal.pbio.0050277
Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants
Abstract
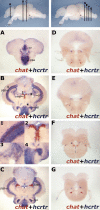
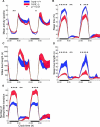
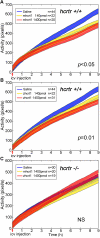
Sleep is a fundamental biological process conserved across the animal kingdom. The study of how sleep regulatory networks are conserved is needed to better understand sleep across evolution. We present a detailed description of a sleep state in adult zebrafish characterized by reversible periods of immobility, increased arousal threshold, and place preference. Rest deprivation using gentle electrical stimulation is followed by a sleep rebound, indicating homeostatic regulation. In contrast to mammals and similarly to birds, light suppresses sleep in zebrafish, with no evidence for a sleep rebound. We also identify a null mutation in the sole receptor for the wake-promoting neuropeptide hypocretin (orexin) in zebrafish. Fish lacking this receptor demonstrate short and fragmented sleep in the dark, in striking contrast to the excessive sleepiness and cataplexy of narcolepsy in mammals. Consistent with this observation, we find that the hypocretin receptor does not colocalize with known major wake-promoting monoaminergic and cholinergic cell groups in the zebrafish. Instead, it colocalizes with large populations of GABAergic neurons, including a subpopulation of Adra2a-positive GABAergic cells in the anterior hypothalamic area, neurons that could assume a sleep modulatory role. Our study validates the use of zebrafish for the study of sleep and indicates molecular diversity in sleep regulatory networks across vertebrates.
Conflict of interest statement
Figures


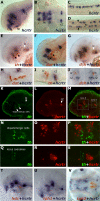
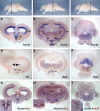
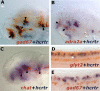
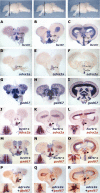

Comment in
-
Let sleeping zebrafish lie: a new model for sleep studies.PLoS Biol. 2007 Oct;5(10):e281. doi: 10.1371/journal.pbio.0050281. Epub 2007 Oct 16. PLoS Biol. 2007. PMID: 20076649 Free PMC article. No abstract available.
References
-
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. - PubMed
-
- Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. - PubMed
-
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster . Science. 2000;287:1834–1837. - PubMed
-
- Cirelli C, Bushey D, Hill S, Huber R, Kreber R, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–1092. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

