PIN: a novel protein involved in IFN-gamma accumulation of NOS-1 in neurons
- PMID: 17941806
- PMCID: PMC2556631
- DOI: 10.1089/dna.2007.0673
PIN: a novel protein involved in IFN-gamma accumulation of NOS-1 in neurons
Abstract
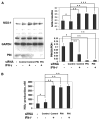
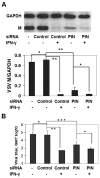
In this study we investigate the role of the protein inhibitor of NOS-1 (PIN) in the interferon-gamma (IFN-gamma)-mediated posttranscriptional accumulation of nitric oxide synthase-1 (NOS-1) and the anti-vesicular stomatitis virus response in neuronal cells. IFN-gamma-induced enhancement of NOS-1 activity is crucial for its antiviral activity in the central nervous system. IFN-gamma treatment of neuronal cells results in an increase of total NOS-1 and decrease of total PIN proteins without alteration in their respective mRNA levels. PIN/NOS-1 complexes decreased after IFN-gamma treatment. Transfection of cells with small interfering RNA (siRNA) for PIN results in a higher constitutive activity of NOS-1 and inhibition of viral replication. IFN-gamma treatment did not change the amount of NOS-1 detectable by Western blot, when PIN is diminished by RNAi treatment. Overexpression of PIN results in lower constitutive NOS-1 expression and activity, and diminishes activation of NOS-1 by IFN-gamma. Our findings indicate that in neurons, IFN-gamma upregulates NOS-1 through proteasomal degradation of PIN.
Figures



Similar articles
-
The role of the proteasome-ubiquitin pathway in regulation of the IFN-gamma mediated anti-VSV response in neurons.J Neuroimmunol. 2006 Dec;181(1-2):34-45. doi: 10.1016/j.jneuroim.2006.07.018. Epub 2006 Sep 7. J Neuroimmunol. 2006. PMID: 16959328 Free PMC article.
-
Posttranscriptional regulation of neuronal nitric oxide synthase expression by IFN-gamma.J Interferon Cytokine Res. 2004 Feb;24(2):141-9. doi: 10.1089/107999004322813381. J Interferon Cytokine Res. 2004. PMID: 14980078
-
Regulation and function of the protein inhibitor of nitric oxide synthase (PIN)/dynein light chain 8 (LC8) in a human mast cell line.Life Sci. 2007 Feb 13;80(10):959-64. doi: 10.1016/j.lfs.2006.11.025. Epub 2006 Nov 22. Life Sci. 2007. PMID: 17169380
-
Interferon-gamma induced type I nitric oxide synthase activity inhibits viral replication in neurons.J Neuroimmunol. 1996 Aug;68(1-2):101-8. doi: 10.1016/0165-5728(96)00083-5. J Neuroimmunol. 1996. PMID: 8784266
-
The role of IFN-gamma in immune responses to viral infections of the central nervous system.Cytokine Growth Factor Rev. 2002 Dec;13(6):441-54. doi: 10.1016/s1359-6101(02)00044-8. Cytokine Growth Factor Rev. 2002. PMID: 12401479 Review.
Cited by
-
DISTINCT MECHANISMS OF INHIBITION OF VSV REPLICATION IN NEURONS MEDIATED BY TYPE I AND TYPE II IFN.Virus Rev Res. 2009 Jan 1;14(2):20-29. Virus Rev Res. 2009. PMID: 20502625 Free PMC article.
-
Disruption of IFN-gamma- mediated antiviral activity in neurons: the role of cannabinoids.Viral Immunol. 2008 Jun;21(2):141-52. doi: 10.1089/vim.2007.0109. Viral Immunol. 2008. PMID: 18570588 Free PMC article.
References
-
- Bender AT, Demady DR, Osawa Y. Ubiquitination of neuronal nitric-oxide synthase in vitro and in vivo. J Biol Chem. 2000;275:17407–17411. - PubMed
-
- Chen N, Reiss CS. Distinct roles of eicosanoids in the immune response to viral encephalitis: or why you should take NSAIDS. Viral Immunol. 2002;15:133–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

