Glutathione mediated regulation of oligomeric structure and functional activity of Plasmodium falciparum glutathione S-transferase
- PMID: 17941979
- PMCID: PMC2110890
- DOI: 10.1186/1472-6807-7-67
Glutathione mediated regulation of oligomeric structure and functional activity of Plasmodium falciparum glutathione S-transferase
Abstract
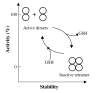
Background: In contrast to many other organisms, the malarial parasite Plasmodium falciparum possesses only one typical glutathione S-transferase. This enzyme, PfGST, cannot be assigned to any of the known GST classes and represents a most interesting target for antimalarial drug development. The PfGST under native conditions forms non-covalently linked higher aggregates with major population (approximately 98%) being tetramer. However, in the presence of 2 mM GSH, a dimer of PfGST is observed. Recently reported study on binding and catalytic properties of PfGST indicated a GSH dependent low-high affinity transition with simultaneous binding of two GSH molecules to PfGST dimer suggesting that GSH binds to low affinity inactive enzyme dimer converting it to high affinity functionally active dimer. In order to understand the role of GSH in tetramer-dimer transition of PfGST as well as in modulation of functional activity of the enzyme, detailed structural, functional and stability studies on recombinant PfGST in the presence and absence of GSH were carried out.
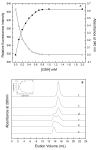
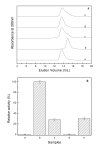
Results: Our data indicate that the dimer - and not the tetramer - is the active form of PfGST, and that substrate saturation is directly paralleled by dissociation of the tetramer. Furthermore, this dissociation is a reversible process indicating that the tetramer-dimer equilibrium of PfGST is defined by the surrounding GSH concentration. Equilibrium denaturation studies show that the PfGST tetramer has significantly higher stability compared to the dimer. The enhanced stability of the tetramer is likely to be due to stronger ionic interactions existing in it.
Conclusion: This is the first report for any GST where an alteration in oligomeric structure and not just small conformational change is observed upon GSH binding to the enzyme. Furthermore we also demonstrate a reversible mechanism of regulation of functional activity of Plasmodium falciparum glutathione S-transferase via GSH induced dissociation of functionally inactive tetramer into active dimers.
Figures


Similar articles
-
Plasmodium falciparum glutathione S-transferase--structural and mechanistic studies on ligand binding and enzyme inhibition.Protein Sci. 2006 Feb;15(2):281-9. doi: 10.1110/ps.051891106. Epub 2005 Dec 29. Protein Sci. 2006. PMID: 16385005 Free PMC article.
-
Calorimetric studies of ligands binding to glutathione S-transferase from the malarial parasite Plasmodium falciparum.Biochemistry. 2013 Mar 19;52(11):1980-9. doi: 10.1021/bi400007g. Epub 2013 Mar 7. Biochemistry. 2013. PMID: 23439010
-
X-ray structure of glutathione S-transferase from the malarial parasite Plasmodium falciparum.Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):13821-6. doi: 10.1073/pnas.2333763100. Epub 2003 Nov 17. Proc Natl Acad Sci U S A. 2003. PMID: 14623980 Free PMC article.
-
Glutathione S-transferase from malarial parasites: structural and functional aspects.Methods Enzymol. 2005;401:241-53. doi: 10.1016/S0076-6879(05)01015-3. Methods Enzymol. 2005. PMID: 16399390 Review.
-
Glutathione--functions and metabolism in the malarial parasite Plasmodium falciparum.Biol Chem. 2003 Apr;384(4):551-66. doi: 10.1515/BC.2003.063. Biol Chem. 2003. PMID: 12751785 Review.
Cited by
-
Tetramerization and cooperativity in Plasmodium falciparum glutathione S-transferase are mediated by atypic loop 113-119.J Biol Chem. 2009 Aug 14;284(33):22133-22139. doi: 10.1074/jbc.M109.015198. Epub 2009 Jun 16. J Biol Chem. 2009. PMID: 19531494 Free PMC article.
-
Characterization of a Highly pH Stable Chi-Class Glutathione S-Transferase from Synechocystis PCC 6803.PLoS One. 2015 May 12;10(5):e0126811. doi: 10.1371/journal.pone.0126811. eCollection 2015. PLoS One. 2015. PMID: 25965384 Free PMC article.
-
Comprehensive analysis of the catalytic and structural properties of a mu-class glutathione s-transferase from Fasciola gigantica.Sci Rep. 2017 Dec 13;7(1):17547. doi: 10.1038/s41598-017-17678-3. Sci Rep. 2017. PMID: 29235505 Free PMC article.
-
Structure of a Highly Active Cephalopod S-crystallin Mutant: New Molecular Evidence for Evolution from an Active Enzyme into Lens-Refractive Protein.Sci Rep. 2016 Aug 8;6:31176. doi: 10.1038/srep31176. Sci Rep. 2016. PMID: 27499004 Free PMC article.
-
Structure-Based Screening of Plasmodium berghei Glutathione S-Transferase Identifies CB-27 as a Novel Antiplasmodial Compound.Front Pharmacol. 2020 Mar 17;11:246. doi: 10.3389/fphar.2020.00246. eCollection 2020. Front Pharmacol. 2020. PMID: 32256353 Free PMC article.
References
-
- Mannervik B, Danielson UH. Glutathione transferases-structure and catalytic activity. CRC Crit Rev Biochem. 1988;23:283–337. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

