R-subunit isoform specificity in protein kinase A: distinct features of protein interfaces in PKA types I and II by amide H/2H exchange mass spectrometry
- PMID: 17942118
- PMCID: PMC3419600
- DOI: 10.1016/j.jmb.2007.09.035
R-subunit isoform specificity in protein kinase A: distinct features of protein interfaces in PKA types I and II by amide H/2H exchange mass spectrometry
Abstract
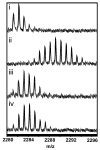
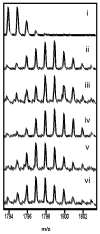
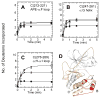
The two isoforms (RI and RII) of the regulatory (R) subunit of cAMP-dependent protein kinase or protein kinase A (PKA) are similar in sequence yet have different biochemical properties and physiological functions. To further understand the molecular basis for R-isoform-specificity, the interactions of the RIIbeta isoform with the PKA catalytic (C) subunit were analyzed by amide H/(2)H exchange mass spectrometry to compare solvent accessibility of RIIbeta and the C subunit in their free and complexed states. Direct mapping of the RIIbeta-C interface revealed important differences between the intersubunit interfaces in the type I and type II holoenzyme complexes. These differences are seen in both the R-subunits as well as the C-subunit. Unlike the type I isoform, the type II isoform complexes require both cAMP-binding domains, and ATP is not obligatory for high affinity interactions with the C-subunit. Surprisingly, the C-subunit mediates distinct, overlapping surfaces of interaction with the two R-isoforms despite a strong homology in sequence and similarity in domain organization. Identification of a remote allosteric site on the C-subunit that is essential for interactions with RII, but not RI subunits, further highlights the considerable diversity in interfaces found in higher order protein complexes mediated by the C-subunit of PKA.
Figures




Similar articles
-
Mapping intersubunit interactions of the regulatory subunit (RIalpha) in the type I holoenzyme of protein kinase A by amide hydrogen/deuterium exchange mass spectrometry (DXMS).J Mol Biol. 2004 Jul 23;340(5):1185-96. doi: 10.1016/j.jmb.2004.05.042. J Mol Biol. 2004. PMID: 15236976
-
Two PKA RIα holoenzyme states define ATP as an isoform-specific orthosteric inhibitor that competes with the allosteric activator, cAMP.Proc Natl Acad Sci U S A. 2019 Aug 13;116(33):16347-16356. doi: 10.1073/pnas.1906036116. Epub 2019 Jul 30. Proc Natl Acad Sci U S A. 2019. PMID: 31363049 Free PMC article.
-
Probing cAMP-dependent protein kinase holoenzyme complexes I alpha and II beta by FT-IR and chemical protein footprinting.Biochemistry. 2004 Feb 24;43(7):1908-20. doi: 10.1021/bi0354435. Biochemistry. 2004. PMID: 14967031
-
Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design.Biochim Biophys Acta. 2008 Jan;1784(1):16-26. doi: 10.1016/j.bbapap.2007.10.002. Epub 2007 Oct 12. Biochim Biophys Acta. 2008. PMID: 17996741 Free PMC article. Review.
-
PKA: lessons learned after twenty years.Biochim Biophys Acta. 2013 Jul;1834(7):1271-8. doi: 10.1016/j.bbapap.2013.03.007. Epub 2013 Mar 25. Biochim Biophys Acta. 2013. PMID: 23535202 Free PMC article. Review.
Cited by
-
Distal recognition sites in substrates are required for efficient phosphorylation by the cAMP-dependent protein kinase.Genetics. 2009 Jun;182(2):529-39. doi: 10.1534/genetics.109.102178. Epub 2009 Apr 13. Genetics. 2009. PMID: 19364808 Free PMC article.
-
Protein Kinase A in neurological disorders.J Neurodev Disord. 2024 Mar 13;16(1):9. doi: 10.1186/s11689-024-09525-0. J Neurodev Disord. 2024. PMID: 38481146 Free PMC article. Review.
-
Steroid and protein ligand binding to cytochrome P450 46A1 as assessed by hydrogen-deuterium exchange and mass spectrometry.Biochemistry. 2009 May 19;48(19):4150-8. doi: 10.1021/bi900168m. Biochemistry. 2009. PMID: 19317426 Free PMC article.
-
Molecular Simulations Reveal an Unresolved Conformation of the Type IA Protein Kinase A Regulatory Subunit and Suggest Its Role in the cAMP Regulatory Mechanism.Biochemistry. 2017 Aug 1;56(30):3885-3888. doi: 10.1021/acs.biochem.7b00461. Epub 2017 Jul 17. Biochemistry. 2017. PMID: 28661131 Free PMC article.
-
Implementing fluorescence anisotropy screening and crystallographic analysis to define PKA isoform-selective activation by cAMP analogs.ACS Chem Biol. 2013 Oct 18;8(10):2164-72. doi: 10.1021/cb400247t. Epub 2013 Sep 10. ACS Chem Biol. 2013. PMID: 23978166 Free PMC article.
References
-
- Francis SH, Corbin JD. Structure and function of cyclic nucleotide-dependent protein kinases. Annu Rev Physiol. 1994;56:237–272. - PubMed
-
- Shabb JB. Physiological substrates of cAMP-dependent protein kinase. Chem Rev. 2001;101(8):2381–2411. - PubMed
-
- Taylor SS, Yang J, Wu J, Haste NM, Radzio-Andzelm E, Anand G. PKA: a portrait of protein kinase dynamics. Biochim Biophys Acta. 2004;1697(1–2):259–269. - PubMed
-
- Hofmann F, Beavo JA, Bechtel PJ, Krebs EG. Comparison of adenosine 3′:5′-monophosphate-dependent protein kinases from rabbit skeletal and bovine heart muscle. J Biol Chem. 1975;250(19):7795–7801. - PubMed
-
- Canaves JM, Taylor SS. Classification and phylogenetic analysis of the cAMP-dependent protein kinase regulatory subunit family. J Mol Evol. 2002;54(1):17–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

