Increased expression of host iron-binding proteins precedes iron accumulation and calcification of primary lung lesions in experimental tuberculosis in the guinea pig
- PMID: 17942369
- PMCID: PMC2271031
- DOI: 10.1016/j.tube.2007.09.002
Increased expression of host iron-binding proteins precedes iron accumulation and calcification of primary lung lesions in experimental tuberculosis in the guinea pig
Abstract
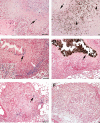
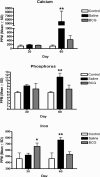
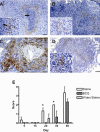
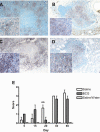
The growth and virulence of Mycobacterium tuberculosis depends on its ability to scavenge host iron, an essential and limited micronutrient in vivo. In this study, we show that ferric iron accumulates both intra- and extra-cellularly in the primary lung lesions of guinea pigs aerosol-infected with the H37Rv strain of M. tuberculosis. Iron accumulated within macrophages at the periphery of the primary granulomatous lesions while extra-cellular ferric iron was concentrated in areas of lesion necrosis. Accumulation of iron within primary lesions was preceded by an increase in expression of heavy chain (H) ferritin, lactoferrin and receptors for transferrin, primarily by macrophages and granulocytes. The increased expression of intra-cellular H ferritin and extra-cellular lactoferrin, more so than transferrin receptor, paralleled the development of necrosis within primary lesions. The deposition of extra-cellular ferric iron within necrotic foci coincided with the accumulation of calcium and phosphorus and other cations in the form of dystrophic calcification. Primary lung lesions from guinea pigs vaccinated with Mycobactrium bovis BCG prior to experimental infection, had reduced iron accumulation as well as H ferritin, lactoferrin and transferrin receptor expression. The amelioration of primary lesion necrosis and dystrophic calcification by BCG vaccination was coincident with the lack of extra-cellular ferric iron and lactoferrin accumulation. These data demonstrate that BCG vaccination ameliorates primary lesion necrosis, dystrophic mineralization and iron accumulation, in part by down-regulating the expression of macrophage H ferritin, lactoferrin and transferrin receptors, in vivo.
Figures
References
-
- Ho RS, Fok JS, Harding GE, Smith DW. Host-parasite relationships in experimental airborne tuberculosis. VII. Fate of Mycobacterium tuberculosis in primary lung lesions and in primary lesion-free lung tissue infected as a result of bacillemia. J. Infect. Dis. 1978;138:237–241. - PubMed
-
- Dubovsky H. A historical basis for modern concepts of the pathogenesis of tuberculosis. S. Afr. Med. J. 1975;49:1105–1110. - PubMed
-
- Smith DW, McMurray DN, Wiegeshaus EH, Grover AA, Harding GE. Host-parasite relationships in experimental airborne tuberculosis. IV. Early events in the course of infection in vaccinated and nonvaccinated guinea pigs. Am. Rev. Respir. Dis. 1970;102:937–949. - PubMed
-
- Canetti G. The Tubercle Bacillus in the Pulmonary Lesion of Man; Histobacteriology and its bearing on the therapy of pulmonary tuberculosis. 2 ed. Springer Publishing Company, Inc; New York: 1955.
-
- Stead WW, Kerby GR, Schlueter DP, Jordahl CW. The clinical spectrum of primary tuberculosis in adults. confusion with reinfection in the pathogenesis of chronic tuberculosis. Ann. Intern. Med. 1968;68:731–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

