Crystal structure of the non-heme iron dioxygenase PtlH in pentalenolactone biosynthesis
- PMID: 17942405
- PMCID: PMC3010413
- DOI: 10.1074/jbc.M706358200
Crystal structure of the non-heme iron dioxygenase PtlH in pentalenolactone biosynthesis
Abstract
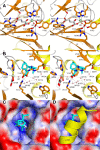
The non-heme iron dioxygenase PtlH from the soil organism Streptomyces avermitilis is a member of the iron(II)/alpha-ketoglutarate-dependent dioxygenase superfamily and catalyzes an essential reaction in the biosynthesis of the sesquiterpenoid antibiotic pentalenolactone. To investigate the structural basis for substrate recognition and catalysis, we have determined the x-ray crystal structure of PtlH in several complexes with the cofactors iron, alpha-ketoglutarate, and the non-reactive enantiomer of the substrate, ent-1-deoxypentalenic acid, in four different crystal forms to up to 1.31 A resolution. The overall structure of PtlH forms a double-stranded barrel helix fold, and the cofactor-binding site for iron and alpha-ketoglutarate is similar to other double-stranded barrel helix fold enzymes. Additional secondary structure elements that contribute to the substrate-binding site in PtlH are not conserved in other double-stranded barrel helix fold enzymes. Binding of the substrate enantiomer induces a reorganization of the monoclinic crystal lattice leading to a disorder-order transition of a C-terminal alpha-helix. The newly formed helix blocks the major access to the active site and effectively traps the bound substrate. Kinetic analysis of wild type and site-directed mutant proteins confirms a critical function of two arginine residues in substrate binding, while simulated docking of the enzymatic reaction product reveals the likely orientation of bound substrate.
Figures


Similar articles
-
Pentalenolactone biosynthesis. Molecular cloning and assignment of biochemical function to PtlH, a non-heme iron dioxygenase of Streptomyces avermitilis.J Am Chem Soc. 2006 May 24;128(20):6566-7. doi: 10.1021/ja061469i. J Am Chem Soc. 2006. PMID: 16704250 Free PMC article.
-
Biochemical and crystallographic investigations into isonitrile formation by a nonheme iron-dependent oxidase/decarboxylase.J Biol Chem. 2021 Jan-Jun;296:100231. doi: 10.1074/jbc.RA120.015932. Epub 2021 Jan 7. J Biol Chem. 2021. PMID: 33361191 Free PMC article.
-
Synthesis of 5-hydroxyectoine from ectoine: crystal structure of the non-heme iron(II) and 2-oxoglutarate-dependent dioxygenase EctD.PLoS One. 2010 May 14;5(5):e10647. doi: 10.1371/journal.pone.0010647. PLoS One. 2010. PMID: 20498719 Free PMC article.
-
Local Charge Distributions, Electric Dipole Moments, and Local Electric Fields Influence Reactivity Patterns and Guide Regioselectivities in α-Ketoglutarate-Dependent Non-heme Iron Dioxygenases.Acc Chem Res. 2022 Jan 4;55(1):65-74. doi: 10.1021/acs.accounts.1c00538. Epub 2021 Dec 17. Acc Chem Res. 2022. PMID: 34915695 Review.
-
Structural studies on 2-oxoglutarate oxygenases and related double-stranded beta-helix fold proteins.J Inorg Biochem. 2006 Apr;100(4):644-69. doi: 10.1016/j.jinorgbio.2006.01.024. Epub 2006 Mar 2. J Inorg Biochem. 2006. PMID: 16513174 Review.
Cited by
-
Crystal structure of prolyl 4-hydroxylase from Bacillus anthracis.Biochemistry. 2010 Jan 12;49(1):124-33. doi: 10.1021/bi901771z. Biochemistry. 2010. PMID: 19947658 Free PMC article.
-
Pentalenic acid is a shunt metabolite in the biosynthesis of the pentalenolactone family of metabolites: hydroxylation of 1-deoxypentalenic acid mediated by CYP105D7 (SAV_7469) of Streptomyces avermitilis.J Antibiot (Tokyo). 2011 Jan;64(1):65-71. doi: 10.1038/ja.2010.135. Epub 2010 Nov 17. J Antibiot (Tokyo). 2011. PMID: 21081950 Free PMC article.
-
Bacterial terpenome.Nat Prod Rep. 2021 May 26;38(5):905-980. doi: 10.1039/d0np00066c. Nat Prod Rep. 2021. PMID: 33169126 Free PMC article. Review.
-
Structural basis for the erythro-stereospecificity of the L-arginine oxygenase VioC in viomycin biosynthesis.FEBS J. 2009 Jul;276(13):3669-82. doi: 10.1111/j.1742-4658.2009.07085.x. Epub 2009 May 26. FEBS J. 2009. PMID: 19490124 Free PMC article.
-
Genome mining of the Streptomyces avermitilis genome and development of genome-minimized hosts for heterologous expression of biosynthetic gene clusters.J Ind Microbiol Biotechnol. 2014 Feb;41(2):233-50. doi: 10.1007/s10295-013-1327-x. Epub 2013 Aug 29. J Ind Microbiol Biotechnol. 2014. PMID: 23990133 Review.
References
-
- Koe BK, Sobin BA, Celmer WD. Antibiot. Annu. 1957:672–675. - PubMed
-
- Keller-Schierlein W, Lemke J, Nyfeler R, Zahner H. Arch. Mikrobiol. 1972;84:301–316. - PubMed
-
- Okazaki T, Enokita R, Torikata A, Inukai M, Takeuchi M, Takahashi S, Arai M. Ann. Rep. Sankyo Res. Lab. 1979;31:94–103.
-
- Takahashi S, Takeuchi M, Arai M, Seto H, Otake N. J Antibiot. 1983;36(3):226–228. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

