Understanding prognostic benefits of exercise and antidepressant therapy for persons with depression and heart disease: the UPBEAT study--rationale, design, and methodological issues
- PMID: 17942470
- PMCID: PMC3677197
- DOI: 10.1177/1740774507083388
Understanding prognostic benefits of exercise and antidepressant therapy for persons with depression and heart disease: the UPBEAT study--rationale, design, and methodological issues
Abstract
Background: Depression is relatively common in patients with coronary heart disease (CHD) and is associated with worse prognosis. Recently there has been interest in evaluating the impact of treating depression on clinical outcomes. Anti-depressant medications have been shown to be safe and efficacious for many patients; exercise also may be effective for treating depression and may also improve cardiopulmonary functioning. However, methodological limitations of previous studies have raised questions about the value of exercise, and no study has compared the effects of exercise with standard anti-depressant medication in depressed cardiac patients.
Purpose: UPBEAT is a randomized clinical trial (RCT) funded by NHLBI to evaluate the effects of sertraline or exercise compared to placebo on depression and biomarkers of cardiovascular risk in patients with CHD and elevated depressive symptoms.
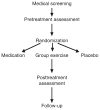
Methods: The UPBEAT study includes 200 stable CHD patients with scores on the Beck Depression Inventory (BDI) > or =9 randomized to 4 months of treatment with aerobic exercise, sertraline, or placebo. The primary outcomes include depressive symptoms determined by clinical ratings on the Hamilton Rating Scale for Depression (HAM-D) and measures of heart rate variability (HRV), baroreflex control (BRC), vascular function (i.e., flow-mediated dilation (FMD)), and measures of inflammation and platelet aggregation.
Results: This article reviews the rationale and design of UPBEAT and addresses several key methodologic issues that were carefully considered in the development of this protocol: the use of a placebo control condition in depressed cardiac patients, study design, and selection of intermediate endpoints or biomarkers of cardiovascular risk.
Limitations: This study is not powered to assess treatment group differences in CHD morbidity and mortality. Intermediate endpoints are not equivalent to 'hard' clinical events and further studies are needed to determine the clinical significance of these biomarkers.
Conclusions: The UPBEAT study is designed to assess the efficacy of exercise in treating depression in cardiac patients and evaluates the impact of treating depression on important biomarkers of cardiovascular risk.
Figures

References
-
- American Heart Association. Heart and stroke statistical update. American Heart Association; Dallas, TX: 2001.
-
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–72. - PubMed
-
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108:1772–78. - PubMed
-
- Barefoot JC. Depression and coronary heart disease. Cardiologia. 1997;42:1245–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

