Endometriosis as a neurovascular condition: estrous variations in innervation, vascularization, and growth factor content of ectopic endometrial cysts in the rat
- PMID: 17942489
- PMCID: PMC2365736
- DOI: 10.1152/ajpregu.00649.2007
Endometriosis as a neurovascular condition: estrous variations in innervation, vascularization, and growth factor content of ectopic endometrial cysts in the rat
Abstract
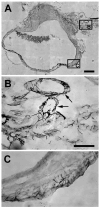
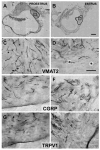
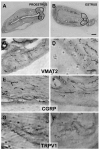
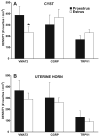
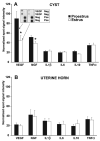
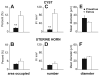
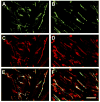
Endometriosis is a poorly understood, estradiol-dependent condition associated with severe pelvic pains and defined by vascularized endometrial growths outside the uterus. Endometriosis is produced in cycling rats by autotransplanting pieces of uterus onto abdominal arteries where they develop into cysts. The surgery induces vaginal and abdominal muscle hyperalgesia, whose severity is greatest in proestrus and nearly absent in estrus. The cysts contain growth factors and cytokines and develop their own sympathetic and sensory C- and Adelta-fiber innervation. Here, we used quantitative immunostaining and protein array analyses to test the hypothesis that the innervation and growth factor/cytokine content of the cysts, but not uterine horn, contribute to proestrous-to-estrous changes in hyperalgesic severity. If so, these characteristics in the cysts, but not the uterine horn, should change with estrous stage. In cysts, the density of sympathetic (but not sensory) neurites and amounts of NGF and VEGF proteins (but not cytokines IL-1, IL-6, IL-10, or TNF-alpha) were greater in proestrus than estrus. These changes were accompanied by vascular changes. Both sympathetic and sensory fibers in both stages colabeled with TrkA, indicating that changes in NGF could act on both afferent and efferent fibers. In contrast with the cysts, no changes occurred in the uterine horn between proestrus and estrus. Together, these results suggest that coordinated proestrous-to-estrous changes in innervation and vascularization of the cysts contribute to similar changes in hyperalgesic severity. The findings also encourage consideration of endometriosis as a neurovascular condition.
Figures
Similar articles
-
Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation.Pain. 2009 Dec 15;147(1-3):255-64. doi: 10.1016/j.pain.2009.09.022. Epub 2009 Oct 12. Pain. 2009. PMID: 19819623 Free PMC article.
-
Innervation of ectopic endometrium in a rat model of endometriosis.Proc Natl Acad Sci U S A. 2004 Jul 27;101(30):11094-8. doi: 10.1073/pnas.0403663101. Epub 2004 Jul 15. Proc Natl Acad Sci U S A. 2004. PMID: 15256593 Free PMC article.
-
Sprouted innervation into uterine transplants contributes to the development of hyperalgesia in a rat model of endometriosis.PLoS One. 2012;7(2):e31758. doi: 10.1371/journal.pone.0031758. Epub 2012 Feb 21. PLoS One. 2012. PMID: 22363725 Free PMC article.
-
Remodeling of uterine innervation.Cell Tissue Res. 2008 Oct;334(1):1-6. doi: 10.1007/s00441-008-0657-x. Epub 2008 Aug 2. Cell Tissue Res. 2008. PMID: 18677514 Review.
-
Mechanism of pain generation for endometriosis-associated pelvic pain.Arch Gynecol Obstet. 2014 Jan;289(1):13-21. doi: 10.1007/s00404-013-3049-8. Epub 2013 Oct 12. Arch Gynecol Obstet. 2014. PMID: 24121693 Review.
Cited by
-
Accumulation of nerve growth factor and its receptors in the uterus and dorsal root ganglia in a mouse model of adenomyosis.Reprod Biol Endocrinol. 2011 Mar 8;9:30. doi: 10.1186/1477-7827-9-30. Reprod Biol Endocrinol. 2011. PMID: 21385399 Free PMC article.
-
Endometriosis-associated pain syndrome: a nurse-led approach.Br J Pain. 2013 Feb;7(1):31-8. doi: 10.1177/2049463713481191. Br J Pain. 2013. PMID: 26516495 Free PMC article.
-
Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: epidemiology, causes, and treatment.Am J Obstet Gynecol. 2018 Apr;218(4):390-400. doi: 10.1016/j.ajog.2017.08.108. Epub 2017 Sep 6. Am J Obstet Gynecol. 2018. PMID: 28888592 Free PMC article. Review.
-
Challenges in uncovering non-invasive biomarkers of endometriosis.Exp Biol Med (Maywood). 2020 Mar;245(5):437-447. doi: 10.1177/1535370220903270. Epub 2020 Feb 4. Exp Biol Med (Maywood). 2020. PMID: 32019326 Free PMC article. Review.
-
Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia.Sci Transl Med. 2011 Sep 21;3(101):101ra91. doi: 10.1126/scitranslmed.3002613. Sci Transl Med. 2011. PMID: 21937756 Free PMC article.
References
-
- Alcazar JL, Garcia-Manero M. Ovarian endometrioma vascularization in women with pelvic pain. Fertil Steril. 2007;87:1271–1276. - PubMed
-
- Ali Z, Raja SN, Wesselmann U, Fuchs PN, Meyer RA, Campbell JN. Intradermal injection of norepinephrine evokes pain in patients with sympathetically maintained pain. Pain. 2000;88:161–168. - PubMed
-
- Anaf V, Simon P, El N I, Fayt I, Simonart T, Buxant F, Noel JC. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002;17:1895–1900. - PubMed
-
- Banik RK, Sato J, Yajima H, Mizumura K. Differences between the Lewis and Sprague-Dawley rats in chronic inflammation induced norepinephrine sensitivity of cutaneous C-fiber nociceptors. Neurosci Lett. 2001;299:21–24. - PubMed
-
- Becker CM, Sampson DA, Rupnick MA, Rohan RM, Efstathiou JA, Short SM, Taylor GA, Folkman J, D'Amato RJ. Endostatin inhibits the growth of endometriotic lesions but does not affect fertility. Fertil Steril. 2005;84 2:1144–1155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

