Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88
- PMID: 17942529
- PMCID: PMC2224366
- DOI: 10.1128/JVI.01640-07
Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88
Abstract
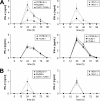
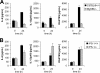
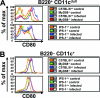
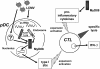
Toll-like receptors (TLRs) and retinoic acid-inducible gene I-like helicases (RLHs) are two major machineries recognizing RNA virus infection of innate immune cells. Intracellular signaling for TLRs and RLHs is mediated by their cytoplasmic adaptors, i.e., MyD88 or TRIF and IPS-1, respectively. In the present study, we investigated the contributions of TLRs and RLHs to the cytotoxic T-lymphocyte (CTL) response by using lymphocytoid choriomeningitis virus (LCMV) as a model virus. The generation of virus-specific cytotoxic T lymphocytes was critically dependent on MyD88 but not on IPS-1. Type I interferons (IFNs) are known to be important for the development of the CTL response to LCMV infection. Serum levels of type I IFNs and proinflammatory cytokines were mainly dependent on the presence of MyD88, although IPS-1(-/-) mice showed a decrease in IFN-alpha levels but not in IFN-beta and proinflammatory cytokine levels. Analysis of Ifna6(+/GFP) reporter mice revealed that plasmacytoid dendritic cells (DCs) are the major source of IFN-alpha in LCMV infection. MyD88(-/-) mice were highly susceptible to LCMV infection in vivo. These results suggest that recognition of LCMV by plasmacytoid DCs via TLRs is responsible for the production of type I IFNs in vivo. Furthermore, the activation of a MyD88-dependent innate mechanism induces a CTL response, which eventually leads to virus elimination.
Figures





References
-
- Aichele, P., H. Unsoeld, M. Koschella, O. Schweier, U. Kalinke, and S. Vucikuja. 2006. Cutting edge: CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J. Immunol. 1764525-4529. - PubMed
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. - PubMed
-
- Beutler, B. 2004. Interferences, questions and possibilities in Toll-like receptor signaling. Nature 230257-263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

