Inhibition of the cyclin-dependent kinases at the beginning of human cytomegalovirus infection specifically alters the levels and localization of the RNA polymerase II carboxyl-terminal domain kinases cdk9 and cdk7 at the viral transcriptosome
- PMID: 17942543
- PMCID: PMC2224385
- DOI: 10.1128/JVI.01681-07
Inhibition of the cyclin-dependent kinases at the beginning of human cytomegalovirus infection specifically alters the levels and localization of the RNA polymerase II carboxyl-terminal domain kinases cdk9 and cdk7 at the viral transcriptosome
Abstract
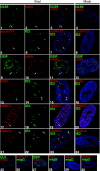
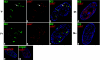
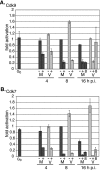
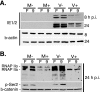
We previously reported that defined components of the host transcription machinery are recruited to human cytomegalovirus immediate-early (IE) transcription sites, including cdk9 and cdk7 (S. Tamrakar, A. J. Kapasi, and D. H. Spector, J. Virol. 79:15477-15493, 2005). In this report, we further document the complexity of this site, referred to as the transcriptosome, through identification of additional resident proteins, including viral UL69 and cellular cyclin T1, Brd4, histone deacetylase 1 (HDAC1), and HDAC2. To examine the role of cyclin-dependent kinases (cdks) in the establishment of this site, we used roscovitine, a specific inhibitor of cdk1, cdk2, cdk7, and cdk9, that alters processing of viral IE transcripts and inhibits expression of viral early genes. In the presence of roscovitine, IE2, cyclin T1, Brd4, HDAC1, and HDAC2 accumulate at the transcriptosome. However, accumulation of cdk9 and cdk7 was specifically inhibited. Roscovitine treatment also resulted in decreased levels of cdk9 and cdk7 RNA. There was a corresponding reduction in cdk9 protein but only a modest decrease in cdk7 protein. However, overexpression of cdk9 does not compensate for the effects of roscovitine on cdk9 localization or viral gene expression. Delaying the addition of roscovitine until 8 h postinfection prevented all of the observed effects of the cdk inhibitor. These data suggest that IE2 and multiple cellular factors needed for viral RNA synthesis accumulate within the first 8 h at the viral transcriptosome and that functional cdk activity is required for the specific recruitment of cdk7 and cdk9 during this time interval.
Figures






Similar articles
-
Human cytomegalovirus infection induces specific hyperphosphorylation of the carboxyl-terminal domain of the large subunit of RNA polymerase II that is associated with changes in the abundance, activity, and localization of cdk9 and cdk7.J Virol. 2005 Dec;79(24):15477-93. doi: 10.1128/JVI.79.24.15477-15493.2005. J Virol. 2005. PMID: 16306619 Free PMC article.
-
Recruitment of cdk9 to the immediate-early viral transcriptosomes during human cytomegalovirus infection requires efficient binding to cyclin T1, a threshold level of IE2 86, and active transcription.J Virol. 2009 Jun;83(11):5904-17. doi: 10.1128/JVI.02651-08. Epub 2009 Mar 18. J Virol. 2009. PMID: 19297489 Free PMC article.
-
Cyclin-dependent kinase activity is required at early times for accurate processing and accumulation of the human cytomegalovirus UL122-123 and UL37 immediate-early transcripts and at later times for virus production.J Virol. 2004 Oct;78(20):11219-32. doi: 10.1128/JVI.78.20.11219-11232.2004. J Virol. 2004. PMID: 15452241 Free PMC article.
-
The involvement of cyclin-dependent kinase 7 (CDK7) and 9 (CDK9) in coordinating transcription and cell cycle checkpoint regulation.Cell Cycle. 2024 Nov-Dec;23(21-24):962-974. doi: 10.1080/15384101.2025.2485844. Epub 2025 Apr 14. Cell Cycle. 2024. PMID: 40223539 Free PMC article. Review.
-
Cyclins that don't cycle--cyclin T/cyclin-dependent kinase-9 determines cardiac muscle cell size.Cell Cycle. 2003 Mar-Apr;2(2):99-104. Cell Cycle. 2003. PMID: 12695656 Review.
Cited by
-
Biologic and immunologic effects of knockout of human cytomegalovirus pp65 nuclear localization signal.Clin Vaccine Immunol. 2009 Jun;16(6):935-43. doi: 10.1128/CVI.00011-09. Epub 2009 Apr 15. Clin Vaccine Immunol. 2009. PMID: 19369477 Free PMC article.
-
Phosphosite Analysis of the Cytomegaloviral mRNA Export Factor pUL69 Reveals Serines with Critical Importance for Recruitment of Cellular Proteins Pin1 and UAP56/URH49.J Virol. 2020 Mar 31;94(8):e02151-19. doi: 10.1128/JVI.02151-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31969433 Free PMC article.
-
Proteomic Interaction Patterns between Human Cyclins, the Cyclin-Dependent Kinase Ortholog pUL97 and Additional Cytomegalovirus Proteins.Viruses. 2016 Aug 18;8(8):219. doi: 10.3390/v8080219. Viruses. 2016. PMID: 27548200 Free PMC article.
-
Functional role of RNA polymerase II and P70 S6 kinase in KCl withdrawal-induced cerebellar granule neuron apoptosis.J Biol Chem. 2015 Feb 27;290(9):5267-79. doi: 10.1074/jbc.M114.575225. Epub 2015 Jan 7. J Biol Chem. 2015. PMID: 25568312 Free PMC article.
-
When cyclin-dependent kinases meet viral infections, including SARS-CoV-2.J Med Virol. 2022 Jul;94(7):2962-2968. doi: 10.1002/jmv.27719. Epub 2022 Mar 23. J Med Virol. 2022. PMID: 35288942 Free PMC article. Review.
References
-
- Ahn, J. H., E. R. Brignole, and G. S. Hayward. 1998. Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML. Mol. Cell. Biol. 184899-4913. - PMC - PubMed
-
- Baek, M.-C., P. M. Krosky, A. Pearson, and D. M. Coen. 2004. Phosphorylation of the RNA polymerase II carboxyl-terminal domain in human cytomegalovirus-infected cells and in vitro by the viral UL97 protein kinase. Virol. 324184-193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

