Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells
- PMID: 17942555
- PMCID: PMC2224386
- DOI: 10.1128/JVI.01910-07
Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells
Abstract
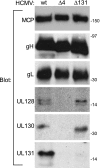
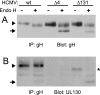
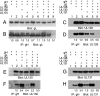
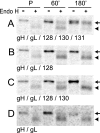
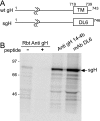
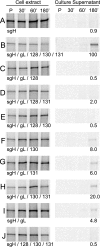
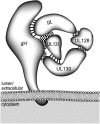
The entry of human cytomegalovirus (HCMV) into biologically relevant epithelial and endothelial cells involves endocytosis followed by low-pH-dependent fusion. This entry pathway is facilitated by the HCMV UL128, UL130, and UL131 proteins, which form one or more complexes with the virion envelope glycoprotein gH/gL. gH/gL/UL128-131 complexes appear to be distinct from the gH/gL/gO complex, which likely facilitates entry into fibroblasts. In order to better understand the assembly and protein-protein interactions of gH/gL/UL128-131 complexes, we generated HCMV mutants lacking UL128-131 proteins and nonreplicating adenovirus vectors expressing gH, gL, UL128, UL130, and UL131. Our results demonstrate that UL128, UL130, and UL131 can each independently assemble onto gH/gL scaffolds. However, the binding of individual UL128-131 proteins onto gH/gL can significantly affect the binding of other proteins; for example, UL128 increased the binding of both UL130 and UL131 to gH/gL. Direct interactions between gH/UL130, UL130/UL131, gL/UL128, and UL128/UL130 were also observed. The export of gH/gL complexes from the endoplasmic reticulum (ER) to the Golgi apparatus and cell surface was dramatically increased when all of UL128, UL130, and UL131 were coexpressed with gH/gL (with or without gO expression). Incorporation of gH/gL complexes into the virion envelope requires transport beyond the ER. Thus, we concluded that UL128, UL130, and UL131 must all bind simultaneously onto gH/gL for the production of complexes that can function in entry into epithelial and endothelial cells.
Figures



References
-
- Adam, E., J. L. Melnick, and M. E. DeBakey. 1997. Cytomegalovirus infection and atherosclerosis. Cent. Eur. J. Public Health 599-106. - PubMed
-
- Adler, B., L. Scrivano, Z. Ruzcics, B. Rupp, C. Sinzger, and U. Koszinowski. 2006. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J. Gen. Virol. 872451-2460. - PubMed
-
- Akter, P., C. Cunningham, B. P. McSharry, A. Dolan, C. Addison, D. J. Dargan, A. F. Hassan-Walker, V. C. Emery, P. D. Griffiths, G. W. Wilkinson, and A. J. Davison. 2003. Two novel spliced genes in human cytomegalovirus. J. Gen. Virol. 841117-1122. - PubMed
-
- Bissinger, A. L., C. Sinzger, E. Kaiserling, and G. Jahn. 2002. Human cytomegalovirus as a direct pathogen: correlation of multiorgan involvement and cell distribution with clinical and pathological findings in a case of congenital inclusion disease. J. Med. Virol. 67200-206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

