Heptad repeat-derived peptides block protease-mediated direct entry from the cell surface of severe acute respiratory syndrome coronavirus but not entry via the endosomal pathway
- PMID: 17942557
- PMCID: PMC2224400
- DOI: 10.1128/JVI.01697-07
Heptad repeat-derived peptides block protease-mediated direct entry from the cell surface of severe acute respiratory syndrome coronavirus but not entry via the endosomal pathway
Abstract
The peptides derived from the heptad repeat (HRP) of severe acute respiratory syndrome coronavirus (SCoV) spike protein (sHRPs) are known to inhibit SCoV infection, yet their efficacies are fairly low. Recently our research showed that some proteases facilitated SCoV's direct entry from the cell surface, resulting in a more efficient infection than the previously known infection via endosomal entry. To compare the inhibitory effect of the sHRP in each pathway, we selected two sHRPs, which showed a strong inhibitory effect on the interaction of two heptad repeats in a rapid and virus-free in vitro assay system. We found that they efficiently inhibited SCoV infection of the protease-mediated cell surface pathway but had little effect on the endosomal pathway. This finding suggests that sHRPs may effectively prevent infection in the lungs, where SCoV infection could be enhanced by proteases produced in this organ. This is the first observation that HRP exhibits different effects on virus that takes the endosomal pathway and virus that enters directly from the cell surface.
Figures

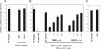
Similar articles
-
Inhibition of Ebola virus entry by a C-peptide targeted to endosomes.J Biol Chem. 2011 May 6;286(18):15854-61. doi: 10.1074/jbc.M110.207084. Epub 2011 Mar 16. J Biol Chem. 2011. PMID: 21454542 Free PMC article.
-
Lysosomal Proteases Are a Determinant of Coronavirus Tropism.J Virol. 2018 Nov 27;92(24):e01504-18. doi: 10.1128/JVI.01504-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30258004 Free PMC article.
-
Protease-mediated entry via the endosome of human coronavirus 229E.J Virol. 2009 Jan;83(2):712-21. doi: 10.1128/JVI.01933-08. Epub 2008 Oct 29. J Virol. 2009. PMID: 18971274 Free PMC article.
-
Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection.Proc Natl Acad Sci U S A. 2005 Aug 30;102(35):12543-7. doi: 10.1073/pnas.0503203102. Epub 2005 Aug 22. Proc Natl Acad Sci U S A. 2005. PMID: 16116101 Free PMC article.
-
MERS-CoV spike protein: a key target for antivirals.Expert Opin Ther Targets. 2017 Feb;21(2):131-143. doi: 10.1080/14728222.2017.1271415. Epub 2016 Dec 21. Expert Opin Ther Targets. 2017. PMID: 27936982 Free PMC article. Review.
Cited by
-
Potential vaccines and post-exposure treatments for filovirus infections.Viruses. 2012 Sep;4(9):1619-50. doi: 10.3390/v4091619. Epub 2012 Sep 21. Viruses. 2012. PMID: 23170176 Free PMC article. Review.
-
Coronavirus membrane fusion mechanism offers a potential target for antiviral development.Antiviral Res. 2020 Jun;178:104792. doi: 10.1016/j.antiviral.2020.104792. Epub 2020 Apr 6. Antiviral Res. 2020. PMID: 32272173 Free PMC article. Review.
-
Recent Progress in Torovirus Molecular Biology.Viruses. 2021 Mar 8;13(3):435. doi: 10.3390/v13030435. Viruses. 2021. PMID: 33800523 Free PMC article. Review.
-
Incorporation of spike and membrane glycoproteins into coronavirus virions.Viruses. 2015 Apr 3;7(4):1700-25. doi: 10.3390/v7041700. Viruses. 2015. PMID: 25855243 Free PMC article. Review.
-
Potential chimeric peptides to block the SARS-CoV-2 spike receptor-binding domain.F1000Res. 2020 Jun 9;9:576. doi: 10.12688/f1000research.24074.1. eCollection 2020. F1000Res. 2020. PMID: 32802318 Free PMC article.
References
-
- Bosch, B. J., B. E. Martina, R. Van Der Zee, J. Lepault, B. J. Haijema, C. Versluis, A. J. Heck, R. De Groot, A. D. Osterhaus, and P. J. Rottier. 2004. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. USA 1018455-8460. - PMC - PubMed
-
- Drosten, C., S. Gunther, W. Preiser, S. van der Werf, H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 3481967-1976. - PubMed

