Altered pathogenesis of porcine respiratory coronavirus in pigs due to immunosuppressive effects of dexamethasone: implications for corticosteroid use in treatment of severe acute respiratory syndrome coronavirus
- PMID: 17942563
- PMCID: PMC2168842
- DOI: 10.1128/JVI.01702-07
Altered pathogenesis of porcine respiratory coronavirus in pigs due to immunosuppressive effects of dexamethasone: implications for corticosteroid use in treatment of severe acute respiratory syndrome coronavirus
Abstract
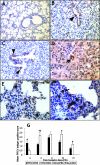
The pathogenesis and optimal treatments for severe acute respiratory syndrome (SARS) are unclear, although corticosteroids were used to reduce lung and systemic inflammation. Because the pulmonary pathology of porcine respiratory coronavirus (PRCV) in pigs resembles SARS, we used PRCV as a model to clarify the effects of the corticosteroid dexamethasone (DEX) on coronavirus (CoV)-induced pneumonia. Conventional weaned pigs (n = 130) in one of four groups (PRCV/phosphate-buffered saline [PBS] [n = 41], PRCV/DEX [n = 41], mock/PBS [n = 23], and mock/DEX [n = 25]) were inoculated intranasally and intratracheally with the ISU-1 strain of PRCV (1 x 10(7) PFU) or cell culture medium. DEX was administered (once daily, 2 mg/kg of body weight/day, intramuscularly) from postinoculation day (PID) 1 to 6. In PRCV/DEX pigs, significantly milder pneumonia, fewer PRCV-positive cells, and lower viral RNA titers were present in lungs early at PID 2; however, at PID 4, 10, and 21, severe bronchointerstitial pneumonia, significantly higher numbers of PRCV-positive cells, and higher viral RNA titers were observed compared to results for PRCV/PBS pigs. Significantly lower numbers of CD2(+), CD3(+), CD4(+), and CD8(+) T cells were also observed in lungs of PRCV/DEX pigs than in those of PRCV/PBS pigs at PID 8 and 10, coincident with fewer gamma interferon (IFN-gamma)-secreting cells in the tracheobronchial lymph nodes as determined by enzyme-linked immunospot assay. Our results confirm that DEX treatment alleviates PRCV pneumonia early (PID 2) in the infection but continued use through PID 6 exacerbates later stages of infection (PID 4, 10, and 21), possibly by decreasing cellular immune responses in the lungs (IFN-gamma-secreting T cells), thereby creating an environment for more-extensive viral replication. These data have potential implications for corticosteroid use with SARS-CoV patients and suggest a precaution against prolonged use based on their unproven efficacy in humans, including possible detrimental secondary effects.
Figures
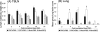
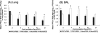

Similar articles
-
Cytokine responses in porcine respiratory coronavirus-infected pigs treated with corticosteroids as a model for severe acute respiratory syndrome.J Virol. 2008 May;82(9):4420-8. doi: 10.1128/JVI.02190-07. Epub 2008 Feb 20. J Virol. 2008. PMID: 18287230 Free PMC article.
-
Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections.J Gen Virol. 2009 Nov;90(Pt 11):2713-2723. doi: 10.1099/vir.0.014001-0. Epub 2009 Aug 5. J Gen Virol. 2009. PMID: 19656969 Free PMC article.
-
Porcine reproductive and respiratory syndrome virus-induced immunosuppression exacerbates the inflammatory response to porcine respiratory coronavirus in pigs.Viral Immunol. 2010 Oct;23(5):457-66. doi: 10.1089/vim.2010.0051. Viral Immunol. 2010. PMID: 20883160 Free PMC article.
-
Comparative Pathogenesis of Bovine and Porcine Respiratory Coronaviruses in the Animal Host Species and SARS-CoV-2 in Humans.J Clin Microbiol. 2020 Jul 23;58(8):e01355-20. doi: 10.1128/JCM.01355-20. Print 2020 Jul 23. J Clin Microbiol. 2020. PMID: 32522830 Free PMC article. Review.
-
Porcine innate and adaptative immune responses to influenza and coronavirus infections.Ann N Y Acad Sci. 2006 Oct;1081(1):130-6. doi: 10.1196/annals.1373.014. Ann N Y Acad Sci. 2006. PMID: 17135502 Free PMC article. Review.
Cited by
-
Assessment of the efficacy of two novel DNA vaccine formulations against highly pathogenic Porcine Reproductive and Respiratory Syndrome Virus.Sci Rep. 2017 Feb 3;7:41886. doi: 10.1038/srep41886. Sci Rep. 2017. PMID: 28157199 Free PMC article.
-
The pig: a model for human infectious diseases.Trends Microbiol. 2012 Jan;20(1):50-7. doi: 10.1016/j.tim.2011.11.002. Epub 2011 Dec 5. Trends Microbiol. 2012. PMID: 22153753 Free PMC article. Review.
-
Impact of Corticosteroid Administration within 7 Days of the Hospitalization for Influenza Pneumonia with Respiratory Failure: A Propensity Score Analysis Using a Nationwide Administrative Database.J Clin Med. 2021 Jan 31;10(3):494. doi: 10.3390/jcm10030494. J Clin Med. 2021. PMID: 33572558 Free PMC article.
-
Efficacy and safety of azvudine versus nirmatrelvir/ritonavir in cancer patients with COVID-19.Sci Rep. 2025 Mar 31;15(1):11022. doi: 10.1038/s41598-025-85677-w. Sci Rep. 2025. PMID: 40164617 Free PMC article.
-
Nitric oxide is elicited and inhibits viral replication in pigs infected with porcine respiratory coronavirus but not porcine reproductive and respiratory syndrome virus.Vet Immunol Immunopathol. 2010 Aug 15;136(3-4):335-9. doi: 10.1016/j.vetimm.2010.03.022. Epub 2010 Apr 1. Vet Immunol Immunopathol. 2010. PMID: 20409593 Free PMC article.
References
-
- Beasley, M. B., T. J. Franks, J. R. Galvin, B. Gochuico, and W. D. Travis. 2002. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch. Pathol. Lab. Med. 126:1064-1070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

