The function of mitochondria in presynaptic development at the neuromuscular junction
- PMID: 17942598
- PMCID: PMC2174173
- DOI: 10.1091/mbc.e07-05-0515
The function of mitochondria in presynaptic development at the neuromuscular junction
Abstract
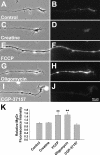
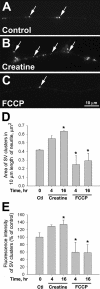
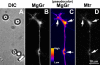
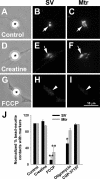
Mitochondria with high membrane potential (DeltaPsi(m)) are enriched in the presynaptic nerve terminal at vertebrate neuromuscular junctions, but the exact function of these localized synaptic mitochondria remains unclear. Here, we investigated the correlation between mitochondrial DeltaPsi(m) and the development of synaptic specializations. Using mitochondrial DeltaPsi(m)-sensitive probe JC-1, we found that DeltaPsi(m) in Xenopus spinal neurons could be reversibly elevated by creatine and suppressed by FCCP. Along naïve neurites, preexisting synaptic vesicle (SV) clusters were positively correlated with mitochondrial DeltaPsi(m), suggesting a potential regulatory role of mitochondrial activity in synaptogenesis. Indicating a specific role of mitochondrial activity in presynaptic development, mitochondrial ATP synthase inhibitor oligomycin, but not mitochondrial Na(+)/Ca(2+) exchanger inhibitor CGP-37157, inhibited the clustering of SVs induced by growth factor-coated beads. Local F-actin assembly induced along spinal neurites by beads was suppressed by FCCP or oligomycin. Our results suggest that a key role of presynaptic mitochondria is to provide ATP for the assembly of actin cytoskeleton involved in the assembly of the presynaptic specialization including the clustering of SVs and mitochondria themselves.
Figures



References
-
- Ankarcrona M., Dypbukt J. M., Bonfoco E., Zhivotovsky B., Orrenius S., Lipton S. A., Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. - PubMed
-
- Baron K. T., Thayer S. A. CGP37157 modulates mitochondrial Ca2+ homeostasis in cultured rat dorsal root ganglion neurons. Eur. J. Pharmacol. 1997;340:295–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

