Enhancement of serological immune responses to foot-and-mouth disease vaccine by a supplement made of extract of cochinchina momordica seeds
- PMID: 17942610
- PMCID: PMC2168389
- DOI: 10.1128/CVI.00339-07
Enhancement of serological immune responses to foot-and-mouth disease vaccine by a supplement made of extract of cochinchina momordica seeds
Abstract
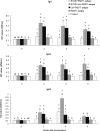
Foot-and-mouth disease (FMD) is a highly contagious disease affecting cloven-hoofed animals. Vaccination against FMD is a routine practice in many countries where the disease is endemic. This study was designed first to investigate the extract of the seeds of Momordica cochinchinensis (Lour.) Spreng. (ECMS) for its adjuvant effect on vaccination of inactivated FMDV antigens in a guinea pig model and then to evaluate the supplement of ECMS in oil-emulsified FMD vaccines for its immunopotentiation in pigs. The results indicated that ECMS and oil emulsion act synergistically as adjuvants to promote the production of FMDV- and VP1-specific immunoglobulin G (IgG) and subclasses in guinea pigs. A supplement of ECMS in a commercial FMD vaccine significantly enhanced FMDV-specific indirect hemagglutination assay titers as well as VP1-specific IgG and subclasses in pigs. Therefore, ECMS could be an alternative approach to improving swine FMD vaccination when the vaccine is poor to induce an effective immune response.
Figures

References
-
- Bachrach, H. L., D. O. Morgan, P. D. McKercher, D. M. Moore, and B. H. Robertson. 1982. Foot-and-mouth disease virus: immunogenicity and structure of fragments derived from capsid protein VP3 and of virus containing cleaved VP3. Vet. Microbiol. 7:85-96. - PubMed
-
- Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Brown, F. 1995. Antibody recognition and neutralisation of foot-and-mouth disease. Semin. Virol. 6:243-248.
-
- Chinese Pharmaceutical Codex Evaluation Committee (ed.). 2005. Mu Bie Zi (Semen momordicae), p. 44. Chinese pharmaceutical codex edition 2005, part I. Chemical Industry Press, Beijing, China.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

