Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy
- PMID: 17942672
- PMCID: PMC2040401
- DOI: 10.1073/pnas.0708148104
Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy
Abstract
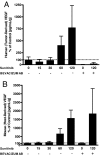
Cancer patients treated with antiangiogenic multitargeted receptor tyrosine kinase (RTK) inhibitors show increased levels of plasma VEGF and placental growth factor and decreased levels of soluble VEGF receptor-2, thus implicating these overall changes as a possible class effect of such drugs and raising the possibility of their exploitation as surrogate biomarkers for pharmacodynamic drug activity/exposure and patient benefit. A postulated mechanism for these changes is that they are tumor-dependent, resulting from drug-induced decreases in vascular function, increases in tumor hypoxia, and changes in hypoxia-regulated genes. However, here we report that an identical pattern of change is observed in normal nontumor-bearing mice treated with SU11248/sunitinib, a small-molecule inhibitor of VEGF and PDGF RTKs. The changes were dose-dependent, plateaued after 4 days of consecutive treatment, reversed after discontinuation of therapy, and correlated with antitumor activity. Altered protein expression was found in a broad variety of tissues, and dose-dependent elevations were observed of several plasma proteins previously unassociated with this class of inhibitor, including G-CSF, SDF-1alpha, SCF, and osteopontin. Our results suggest that observed sunitinib-induced molecular plasma changes, including those both directly and indirectly targeted by drug, represent a systemic tumor-independent response to therapy and may correlate with the most efficacious antitumor doses, potentially having utility for defining the optimal biologic dose range for this drug class but not as predictive markers of tumor response or clinical benefit. They may also be relevant to drug-associated toxicities, drug resistance, and observed rapid tumor (re)growth seen after cessation of therapy.
Conflict of interest statement
Conflict of interest statement: J.G.C. works at Pfizer.
Figures




References
-
- Woude GF, Kelloff GJ, Ruddon RW, Koo HM, Sigman CC, Barrett JC, Day RW, Dicker AP, Kerbel RS, Parkinson DR, et al. Clin Cancer Res. 2004;10:3897–3907. - PubMed
-
- Kelloff GJ, Bast RC, Jr, Coffey DS, D'Amico AV, Kerbel RS, Park JW, Ruddon RW, Rustin GJ, Schilsky RL, Sigman CC, et al. Clin Cancer Res. 2004;10:3881–3884. - PubMed
-
- Eskens FA, Verweij J. Eur J Cancer. 2006;42:3127–3129. - PubMed
-
- Ebos JML, Bocci G, Man S, Thorpe PE, Hicklin DJ, Zhou D, Jia X, Kerbel RS. Mol Cancer Res. 2004;2:315–326. - PubMed
-
- Drevs J, Zirrgiebel U, Schmidt-Gersbach CI, Mross K, Medinger M, Lee L, Pinheiro J, Wood J, Thomas AL, Unger C, et al. Ann Oncol. 2005;16:558–565. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

