Linking folding with aggregation in Alzheimer's beta-amyloid peptides
- PMID: 17942695
- PMCID: PMC2040412
- DOI: 10.1073/pnas.0703832104
Linking folding with aggregation in Alzheimer's beta-amyloid peptides
Abstract
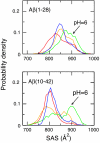
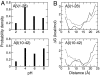
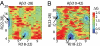
Growing evidence suggests that the beta-amyloid (Abeta) peptides of Alzheimer's disease are generated in early endosomes and that small oligomers are the principal toxic species. We sought to understand whether and how the solution pH, which is more acidic in endosomes than the extracellular environment, affects the conformational processes of Abeta. Using constant pH molecular dynamics simulations of two model peptides, Abeta(1-28) and Abeta(10-42), we found that the folding landscape of Abeta is strongly modulated by pH and is most favorable for hydrophobically driven aggregation at pH 6. Thus, our theoretical findings substantiate the possibility that Abeta oligomers develop intracellularly before secretion into the extracellular milieu, where they may disrupt synaptic activity or act as seeds for plaque formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

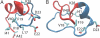

Similar articles
-
Effect of amino-acid substitutions on Alzheimer's amyloid-beta peptide-glycosaminoglycan interactions.Eur J Biochem. 2000 Nov;267(21):6353-61. doi: 10.1046/j.1432-1327.2000.01725.x. Eur J Biochem. 2000. PMID: 11029577
-
Molecular dynamics simulations of low-ordered alzheimer β-amyloid oligomers from dimer to hexamer on self-assembled monolayers.Langmuir. 2011 Dec 20;27(24):14876-87. doi: 10.1021/la2027913. Epub 2011 Nov 22. Langmuir. 2011. PMID: 22077332
-
Stabilization of a beta-hairpin in monomeric Alzheimer's amyloid-beta peptide inhibits amyloid formation.Proc Natl Acad Sci U S A. 2008 Apr 1;105(13):5099-104. doi: 10.1073/pnas.0711731105. Epub 2008 Mar 28. Proc Natl Acad Sci U S A. 2008. PMID: 18375754 Free PMC article.
-
Polymorphism in Alzheimer Abeta amyloid organization reflects conformational selection in a rugged energy landscape.Chem Rev. 2010 Aug 11;110(8):4820-38. doi: 10.1021/cr900377t. Chem Rev. 2010. PMID: 20402519 Free PMC article. Review. No abstract available.
-
Insights into Aβ aggregation: a molecular dynamics perspective.Curr Top Med Chem. 2012;12(22):2596-610. doi: 10.2174/1568026611212220012. Curr Top Med Chem. 2012. PMID: 23339310 Review.
Cited by
-
Molecular Dynamics Simulation for All.Neuron. 2018 Sep 19;99(6):1129-1143. doi: 10.1016/j.neuron.2018.08.011. Neuron. 2018. PMID: 30236283 Free PMC article. Review.
-
Structural diversity of Alzheimer's disease amyloid-β dimers and their role in oligomerization and fibril formation.J Alzheimers Dis. 2014;39(3):583-600. doi: 10.3233/JAD-131589. J Alzheimers Dis. 2014. PMID: 24240640 Free PMC article.
-
Effects of Zn2+ binding on the structural and dynamic properties of amyloid β peptide associated with Alzheimer's disease: Asp1 or Glu11?ACS Chem Neurosci. 2013 Nov 20;4(11):1458-68. doi: 10.1021/cn4001445. Epub 2013 Sep 13. ACS Chem Neurosci. 2013. PMID: 23947440 Free PMC article.
-
Optimization of the GBMV2 implicit solvent force field for accurate simulation of protein conformational equilibria.J Comput Chem. 2017 Jun 15;38(16):1332-1341. doi: 10.1002/jcc.24734. Epub 2017 Apr 11. J Comput Chem. 2017. PMID: 28397268 Free PMC article.
-
Probing pH-dependent dissociation of HdeA dimers.J Am Chem Soc. 2011 Dec 7;133(48):19393-8. doi: 10.1021/ja2060066. Epub 2011 Nov 9. J Am Chem Soc. 2011. PMID: 22026371 Free PMC article.
References
-
- Dobson CM. Nature. 2003;426:884–890. - PubMed
-
- Dyson HJ, Rance M, Houghten RA, Wright PE, Lerner RA. J Mol Biol. 1988;201:201–217. - PubMed
-
- Barrow CJ, Yasuda A, Kenny PTM, Zagorski MG. J Mol Biol. 1992;225:1075–1093. - PubMed
-
- Hou L, Shao H, Zhang Y, Li H, Menon NK, Neuhaus EB, Brewer JM, Byeon I-JL, Ray DG, Vitek MP, et al. J Am Chem Soc. 2004;126:1992–2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

