Linking folding with aggregation in Alzheimer's beta-amyloid peptides
- PMID: 17942695
- PMCID: PMC2040412
- DOI: 10.1073/pnas.0703832104
Linking folding with aggregation in Alzheimer's beta-amyloid peptides
Abstract
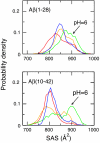
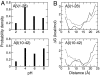
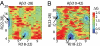
Growing evidence suggests that the beta-amyloid (Abeta) peptides of Alzheimer's disease are generated in early endosomes and that small oligomers are the principal toxic species. We sought to understand whether and how the solution pH, which is more acidic in endosomes than the extracellular environment, affects the conformational processes of Abeta. Using constant pH molecular dynamics simulations of two model peptides, Abeta(1-28) and Abeta(10-42), we found that the folding landscape of Abeta is strongly modulated by pH and is most favorable for hydrophobically driven aggregation at pH 6. Thus, our theoretical findings substantiate the possibility that Abeta oligomers develop intracellularly before secretion into the extracellular milieu, where they may disrupt synaptic activity or act as seeds for plaque formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

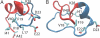

References
-
- Dobson CM. Nature. 2003;426:884–890. - PubMed
-
- Dyson HJ, Rance M, Houghten RA, Wright PE, Lerner RA. J Mol Biol. 1988;201:201–217. - PubMed
-
- Barrow CJ, Yasuda A, Kenny PTM, Zagorski MG. J Mol Biol. 1992;225:1075–1093. - PubMed
-
- Hou L, Shao H, Zhang Y, Li H, Menon NK, Neuhaus EB, Brewer JM, Byeon I-JL, Ray DG, Vitek MP, et al. J Am Chem Soc. 2004;126:1992–2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

