Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways
- PMID: 17942699
- PMCID: PMC2040425
- DOI: 10.1073/pnas.0702761104
Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways
Abstract
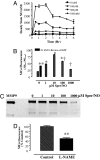
Matrix metalloproteinases (MMPs) are of central importance in the proteolytic remodeling of matrix and the generation of biologically active molecules. MMPs are distinguished by a conserved catalytic domain containing a zinc ion, as well as a prodomain that regulates enzyme activation by modulation of a cysteine residue within that domain. Because nitric oxide (NO) and derived reactive nitrogen species target zinc ions and cysteine thiols, we assessed the ability of NO to regulate MMPs. A dose-dependent, biphasic regulatory effect of NO on the activity of MMPs (MMP-9, -1, and -13) secreted from murine macrophages was observed. Low exogenous NO perturbed MMP/tissue inhibitor of metalloproteinase (TIMP)-1 levels by enhancing MMP activity and suppressing the endogenous inhibitor TIMP-1. This was cGMP-dependent, as confirmed by the cGMP analog 8-bromo-cGMP, as well as by the NO-soluble guanylyl cyclase-cGMP signaling inhibitor thrombospondin-1. Exposure of purified latent MMP-9 to exogenous NO demonstrated a concentration-dependent activation and inactivation of the enzyme, which occurred at higher NO flux. These chemical reactions occurred at concentrations similar to that of activated macrophages. Importantly, these results suggest that NO regulation of MMP-9 secreted from macrophages may occur chemically by reactive nitrogen species-mediated protein modification, biologically through soluble guanylyl-cyclase-dependent modulation of the MMP-9/TIMP-1 balance, or proteolytically through regulation of MMP-1 and -13, which can cleave the prodomain of MMP-9. Furthermore, when applied in a wound model, conditioned media exhibiting peak MMP activity increased vascular cell migration that was MMP-9-dependent, suggesting that MMP-9 is a key physiologic mediator of the effects of NO in this model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
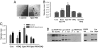
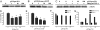
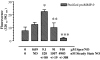
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

