The heat shock response and chaperones/heat shock proteins in brain tumors: surface expression, release, and possible immune consequences
- PMID: 17942716
- PMCID: PMC6673020
- DOI: 10.1523/JNEUROSCI.3588-07.2007
The heat shock response and chaperones/heat shock proteins in brain tumors: surface expression, release, and possible immune consequences
Abstract
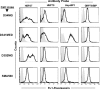
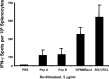
The heat shock response is a highly conserved "stress response" mechanism used by cells to protect themselves from potentially damaging insults. It often involves the upregulated expression of chaperone and heat shock proteins (HSPs) to prevent damage and aggregation at the proteome level. Like most cancers, brain tumor cells often overexpress chaperones/HSPs, probably because of the stressful atmosphere in which tumors reside, but also because of the benefits of HSP cytoprotection. However, the cellular dynamics and localization of HSPs in either stressed or unstressed conditions has not been studied extensively in brain tumor cells. We have examined the changes in HSP expression and in cell surface/extracellular localization of selected brain tumor cell lines under heat shock or normal environments. We herein report that brain tumor cell lines have considerable heat shock responses or already high constitutive HSP levels; that those cells express various HSPs, chaperones, and at least one cochaperone on their cell surfaces; and that HSPs may be released into the extracellular environment, possibly as exosome vesicular content. In studies with a murine astrocytoma cell line, heat shock dramatically reduces tumorigenicity, possibly by an immune mechanism. Additional evidence indicative of an HSP-driven immune response comes from immunization studies using tumor-derived chaperone protein vaccines, which lead to antigen-specific immune responses and reduced tumor burden in treated mice. The heat shock response and HSPs in brain tumor cells may represent an area of vulnerability in our attempts to treat these recalcitrant and deadly tumors.
Figures








References
-
- Arap MA, Lahdenranta J, Mintz PJ, Hajitou A, Sarkis AS, Arap W, Pasqualini R. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6:275–284. - PubMed
-
- Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442–451. - PubMed
-
- Bullard DE, Schold SC, Jr, Bigner SH, Bigner DD. Growth and chemotherapeutic response in athymic mice of tumors arising from human glioma-derived cell lines. J Neuropathol Exp Neurol. 1981;40:410–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
