Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation
- PMID: 17942730
- PMCID: PMC6673037
- DOI: 10.1523/JNEUROSCI.0723-07.2007
Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation
Abstract
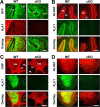
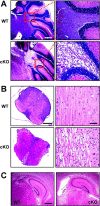
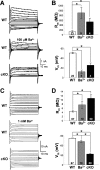
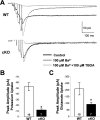
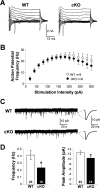
During neuronal activity, extracellular potassium concentration ([K+]out) becomes elevated and, if uncorrected, causes neuronal depolarization, hyperexcitability, and seizures. Clearance of K+ from the extracellular space, termed K+ spatial buffering, is considered to be an important function of astrocytes. Results from a number of studies suggest that maintenance of [K+]out by astrocytes is mediated by K+ uptake through the inward-rectifying Kir4.1 channels. To study the role of this channel in astrocyte physiology and neuronal excitability, we generated a conditional knock-out (cKO) of Kir4.1 directed to astrocytes via the human glial fibrillary acidic protein promoter gfa2. Kir4.1 cKO mice die prematurely and display severe ataxia and stress-induced seizures. Electrophysiological recordings revealed severe depolarization of both passive astrocytes and complex glia in Kir4.1 cKO hippocampal slices. Complex cell depolarization appears to be a direct consequence of Kir4.1 removal, whereas passive astrocyte depolarization seems to arise from an indirect developmental process. Furthermore, we observed a significant loss of complex glia, suggestive of a role for Kir4.1 in astrocyte development. Kir4.1 cKO passive astrocytes displayed a marked impairment of both K+ and glutamate uptake. Surprisingly, membrane and action potential properties of CA1 pyramidal neurons, as well as basal synaptic transmission in the CA1 stratum radiatum appeared unaffected, whereas spontaneous neuronal activity was reduced in the Kir4.1 cKO. However, high-frequency stimulation revealed greatly elevated posttetanic potentiation and short-term potentiation in Kir4.1 cKO hippocampus. Our findings implicate a role for glial Kir4.1 channel subunit in the modulation of synaptic strength.
Figures



References
-
- Akopian G, Kuprijanova E, Kressin K, Steinhuser C. Analysis of ion channel expression by astrocytes in red nucleus brain stem slices of the rat. Glia. 1997;19:234–246. - PubMed
-
- Balestrino M, Aitken PG, Somjen GG. The effects of moderate changes of extracellular K+ and Ca2+ on synaptic and neural function in the CA1 region of the hippocampal slice. Brain Res. 1986;377:229–239. - PubMed
-
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
