Role of kinase suppressor of Ras-1 in neuronal survival signaling by extracellular signal-regulated kinase 1/2
- PMID: 17942733
- PMCID: PMC6673027
- DOI: 10.1523/JNEUROSCI.3473-07.2007
Role of kinase suppressor of Ras-1 in neuronal survival signaling by extracellular signal-regulated kinase 1/2
Abstract
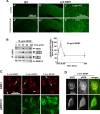
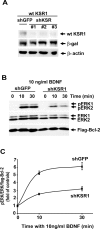
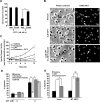
Scaffolding proteins including kinase suppressor of Ras-1 (KSR1) determine specificity of signaling by extracellular signal-regulated kinase 1/2 (ERK1/2), enabling it to couple diverse extracellular stimuli to various cellular responses. The scaffolding protein(s) that contributes to ERK1/2-mediated neuronal survival has not yet been identified. In cultured rat cortical neurons, BDNF activates ERK1/2 to enhance neuronal survival by suppressing DNA damage- or trophic deprivation-induced apoptosis. Here we report that in this system, BDNF increased KSR1 association with activated ERK1/2, whereas KSR1 knockdown with a short hairpin (sh) RNA reduced BDNF-mediated activation of ERK1/2 and protection against a DNA-damaging drug, camptothecin (CPT). In contrast, BDNF suppression of trophic deprivation-induced apoptosis was unaffected by shKSR1 although blocked by shERK1/2. Also, overexpression of KSR1 enhanced BDNF protection against CPT. Therefore, KSR1 is specifically involved in antigenotoxic activation of ERK1/2 by BDNF. To test whether KSR1 contributes to ERK1/2 activation by other neuroprotective stimuli, we used a cAMP-elevating drug, forskolin. In cortical neurons, ERK1/2 activation by forskolin was protein kinase A (PKA) dependent but TrkB (receptor tyrosine kinase B) independent and was accompanied by the increased association between KSR1 and active ERK1/2. Forskolin suppressed CPT-induced apoptosis in a KSR1 and ERK1/2-dependent manner. Inhibition of PKA abolished forskolin protection, whereas selective PKA activation resulted in an ERK1/2- and KSR1-mediated decrease in apoptosis. Hence, KSR1 is critical for the antiapoptotic activation of ERK1/2 by BDNF or cAMP/PKA signaling. In addition, these novel data indicate that stimulation of cAMP signaling is a candidate neuroprotective strategy to intervene against neurotoxicity of DNA-damaging agents.
Figures







References
-
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. - PubMed
-
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms [see comments] Science. 1999;286:1358–1362. - PubMed
-
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. - PubMed
-
- Cacace AM, Michaud NR, Therrien M, Mathes K, Copeland T, Rubin GM, Morrison DK. Identification of constitutive and ras-inducible phosphorylation sites of KSR: implications for 14-3-3 binding, mitogen-activated protein kinase binding, and KSR overexpression. Mol Cell Biol. 1999;19:229–240. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
