Cyclin-dependent kinase 2/cyclin E complex is involved in p120 catenin (p120ctn)-dependent cell growth control: a new role for p120ctn in cancer
- PMID: 17942908
- PMCID: PMC2695941
- DOI: 10.1158/0008-5472.CAN-07-0233
Cyclin-dependent kinase 2/cyclin E complex is involved in p120 catenin (p120ctn)-dependent cell growth control: a new role for p120ctn in cancer
Abstract
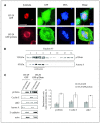
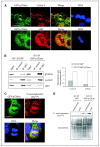
Depending on its cellular localization, p120 catenin (p120ctn) can participate in various processes, such as cadherin-dependent cell-cell adhesion, actin cytoskeleton remodeling, and intracellular trafficking. Recent studies also indicate that p120ctn could regulate cell proliferation and contact inhibition. This report describes a new function of p120ctn in the regulation of cell cycle progression. Overexpression of the p120ctn isoform 3A in human colon adenocarcinoma cells (HT-29) results in cytoplasmic accumulation of the protein, as observed in many tumors. This cytoplasmic increase is correlated with a reduction in proliferation and inhibition of DNA synthesis. Under these conditions, experiments on synchronized cells revealed a prolonged S phase associated with cyclin E stabilization. Both confocal microscopy and biochemical analysis showed that cyclin E and cyclin-dependent kinase 2 colocalized with p120ctn in centrosomes during mitosis. These proteins are associated in a functional complex evidenced by coimmunoprecipitation experiments and the emergence of Thr199-phosphorylated nucleophosmin/B23. Such post-translational modification of this centrosomal target has been shown to trigger the initiation of centrosome duplication. Therefore, p120ctn-mediated accumulation of cyclin E in centrosomes may participate in abnormal amplification of centrosomes and the inhibition of DNA replication, thus leading to aberrant mitosis and polyploidy. Because these modifications are often observed in cancer, p120ctn may represent a new therapeutic target for future therapy.
Figures




Similar articles
-
P120-catenin isoforms 1 and 3 regulate proliferation and cell cycle of lung cancer cells via β-catenin and Kaiso respectively.PLoS One. 2012;7(1):e30303. doi: 10.1371/journal.pone.0030303. Epub 2012 Jan 20. PLoS One. 2012. PMID: 22276175 Free PMC article.
-
Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease.Cancer Res. 2005 Dec 1;65(23):10938-45. doi: 10.1158/0008-5472.CAN-05-1947. Cancer Res. 2005. PMID: 16322241
-
P120ctn overexpression enhances beta-catenin-E-cadherin binding and down regulates expression of survivin and cyclin D1 in BEL-7404 hepatoma cells.World J Gastroenterol. 2006 Feb 28;12(8):1187-91. doi: 10.3748/wjg.v12.i8.1187. World J Gastroenterol. 2006. PMID: 16534869 Free PMC article.
-
Diverse functions of p120ctn in tumors.Biochim Biophys Acta. 2007 Jan;1773(1):78-88. doi: 10.1016/j.bbamcr.2006.08.033. Epub 2006 Aug 30. Biochim Biophys Acta. 2007. PMID: 17030444 Review.
-
p120 catenin and phosphorylation: Mechanisms and traits of an unresolved issue.Biochim Biophys Acta. 2007 Jan;1773(1):47-58. doi: 10.1016/j.bbamcr.2006.06.001. Epub 2006 Jun 17. Biochim Biophys Acta. 2007. PMID: 16904204 Review.
Cited by
-
p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression.Prog Mol Biol Transl Sci. 2013;116:409-32. doi: 10.1016/B978-0-12-394311-8.00018-2. Prog Mol Biol Transl Sci. 2013. PMID: 23481205 Free PMC article. Review.
-
Nuclear p120 catenin is a component of the perichromosomal layer and coordinates sister chromatid segregation during mitosis in lung cancer cells.Cell Death Dis. 2022 Jun 4;13(6):526. doi: 10.1038/s41419-022-04929-z. Cell Death Dis. 2022. PMID: 35660718 Free PMC article.
-
p120 catenin induces opposing effects on tumor cell growth depending on E-cadherin expression.J Cell Biol. 2008 Nov 17;183(4):737-49. doi: 10.1083/jcb.200805113. J Cell Biol. 2008. PMID: 19015320 Free PMC article.
-
P120-catenin isoforms 1 and 3 regulate proliferation and cell cycle of lung cancer cells via β-catenin and Kaiso respectively.PLoS One. 2012;7(1):e30303. doi: 10.1371/journal.pone.0030303. Epub 2012 Jan 20. PLoS One. 2012. PMID: 22276175 Free PMC article.
-
delta-Catenin promotes prostate cancer cell growth and progression by altering cell cycle and survival gene profiles.Mol Cancer. 2009 Mar 10;8:19. doi: 10.1186/1476-4598-8-19. Mol Cancer. 2009. PMID: 19284555 Free PMC article.
References
-
- Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–19. - PubMed
-
- Reynolds AB, Carnahan RH. Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer. Semin Cell Dev Biol. 2004;15:657–63. - PubMed
-
- Waltzer L, Bienz M. The control of β-catenin and TCF during embryonic development and cancer. Cancer Metastasis Rev. 1999;18:231–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

