Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture
- PMID: 1794314
- PMCID: PMC2975574
- DOI: 10.1242/dev.112.2.439
Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture
Abstract
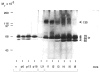
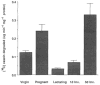
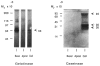
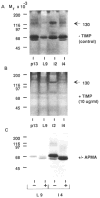
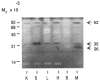
The extracellular matrix (ECM) is an important regulator of mammary epithelial cell function both in vivo and in culture. Substantial remodeling of ECM accompanies the structural changes in the mammary gland during gestation, lactation and involution. However, little is known about the nature of the enzymes and the processes involved. We have characterized and studied the regulation of cell-associated and secreted mammary gland proteinases active at neutral pH that may be involved in degradation of the ECM during the different stages of mammary development. Mammary tissue extracts from virgin and pregnant CD-1 mice resolved by zymography contained three major proteinases of 60K (K = 10(3) Mr), 68K and 70K that degraded denatured collagen. These three gelatinases were completely inhibited by the tissue inhibitor of metalloproteinases. Proteolytic activity was lowest during lactation especially for the 60K gelatinase which was shown to be the activated form of the 68K gelatinase. The activated 60K form decreased prior to parturition but increased markedly after the first two days of involution. An additional gelatin-degrading proteinase of 130K was expressed during the first three days of involution and differed from the other gelatinases by its lack of inhibition by the tissue inhibitor of metalloproteinases. The activity of the casein-degrading proteinases was lowest during lactation. Three caseinolytic activities were detected in mammary tissue extracts. A novel 26K cell-associated caseinase--a serine arginine-esterase--was modulated at different stages of mammary development. The other caseinases, at 92K and a larger than 100K, were not developmentally regulated. To find out which cell type produced the proteinases in the mammary gland, we isolated and cultured mouse mammary epithelial cells. Cells cultured on different substrata produced the full spectrum of gelatinases and caseinases seen in the whole gland thus implicating the epithelial cells as a major source of these enzymes. Analysis of proteinases secreted by cells grown on a reconstituted basement membrane showed that gelatinases were secreted preferentially in the direction of the basement membrane. The temporal pattern of expression of these proteinases and the basal secretion of gelatinases by epithelial cells suggest their involvement in the remodelling of the extracellular matrix during the different stages of mammary development and thus modulation of mammary cell function.
Figures


Similar articles
-
Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways.Development. 1996 Jan;122(1):181-93. doi: 10.1242/dev.122.1.181. Development. 1996. PMID: 8565829 Free PMC article.
-
Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution.J Cell Biol. 1992 Sep;118(5):1271-82. doi: 10.1083/jcb.118.5.1271. J Cell Biol. 1992. PMID: 1512297 Free PMC article.
-
Modulation of neutral matrix metalloproteinases of involuting rat mammary gland by different cations and glycosaminoglycans.J Cell Biochem. 1999 May 1;73(2):218-26. J Cell Biochem. 1999. PMID: 10227385
-
ECM degrading proteases and tissue remodelling in the mammary gland.Bioessays. 2005 Sep;27(9):894-903. doi: 10.1002/bies.20281. Bioessays. 2005. PMID: 16108064 Review.
-
[Mammary gland development: Role of basal myoepithelial cells].J Soc Biol. 2006;200(2):193-8. doi: 10.1051/jbio:2006021. J Soc Biol. 2006. PMID: 17151555 Review. French.
Cited by
-
Stromelysin-3 in the biology of the normal and neoplastic mammary gland.J Mammary Gland Biol Neoplasia. 1996 Apr;1(2):231-40. doi: 10.1007/BF02013646. J Mammary Gland Biol Neoplasia. 1996. PMID: 10887496 Review.
-
Mammary involution and breast cancer risk: transgenic models and clinical studies.J Mammary Gland Biol Neoplasia. 2009 Jun;14(2):181-91. doi: 10.1007/s10911-009-9123-y. Epub 2009 Apr 30. J Mammary Gland Biol Neoplasia. 2009. PMID: 19404726 Free PMC article. Review.
-
Characterization and Comparative Analysis of the Milk Transcriptome in Two Dairy Sheep Breeds using RNA Sequencing.Sci Rep. 2015 Dec 18;5:18399. doi: 10.1038/srep18399. Sci Rep. 2015. PMID: 26677795 Free PMC article.
-
MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis.Cell. 2000 Oct 27;103(3):481-90. doi: 10.1016/s0092-8674(00)00139-2. Cell. 2000. PMID: 11081634 Free PMC article.
-
Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways.Development. 1996 Jan;122(1):181-93. doi: 10.1242/dev.122.1.181. Development. 1996. PMID: 8565829 Free PMC article.
References
-
- Adler RR, Brenner CA, Werb Z. Expression of extracellular matrix-degrading metalloproteinases and metalloproteinase inhibitors is developmentally regulated during endoderm differentiation of embryonal carcinoma cells. Development. 1990;110:211–220. - PubMed
-
- Alexander CM, Werb Z. Proteinases and extracellular matrix remodeling. Current Opinion In Cell Biology. 1989;1:974–982. - PubMed
-
- Anderson RR. Mammary gland. In: Larson BL, editor. Lactation. Ames, IA: Iowa State University Press; 1985. pp. 3–37.
-
- Ballin M, Gomez DE, Sinha CC, Thorgeirsson UP. Ras oncogene mediated induction of a 92 kDa metalloproteinase; strong correlation with the malignant phenotype. Biochem biophys Res Commun. 1988;154:832–838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

