Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers
- PMID: 17943185
- PMCID: PMC2671223
- DOI: 10.1038/sj.jid.5701099
Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers
Abstract
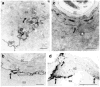
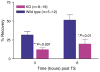
Human epidermis elaborates two small cationic, highly hydrophobic antimicrobial peptides (AMP), beta-defensin 2 (hBD2), and the carboxypeptide cleavage product of human cathelicidin (hCAP18), LL-37, which are co-packaged along with lipids within epidermal lamellar bodies (LBs) before their secretion. Because of their colocalization, we hypothesized that AMP and barrier lipid production could be coregulated by altered permeability barrier requirements. mRNA and immunostainable protein levels for mBD3 and cathelin-related antimicrobial peptide (CRAMP) (murine homologues of hBD2 and LL-37, respectively) increase 1-8 hours after acute permeability barrier disruption and normalize by 24 hours, kinetics that mirror the lipid metabolic response to permeability barrier disruption. Artificial permeability barrier restoration, which inhibits the lipid-synthetic response leading to barrier recovery, blocks the increase in AMP mRNA/protein expression, further evidence that AMP expression is linked to permeability barrier function. Conversely, LB-derived AMPs are also important for permeability barrier homeostasis. Despite an apparent increase in mBD3 protein, CRAMP-/- mice delayed permeability barrier recovery, attributable to defective LB contents and abnormalities in the structure of the lamellar membranes that regulate permeability barrier function. These studies demonstrate that (1) the permeability and antimicrobial barriers are coordinately regulated by permeability barrier requirements and (2) CRAMP is required for permeability barrier homeostasis.
Conflict of interest statement
The authors state no conflict of interest.
Figures



References
-
- Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol. 2001;117:309–17. - PubMed
-
- Bibel DJ, Aly R, Shinefield HR. Antimicrobial activity of sphingosines. J Invest Dermatol. 1992;98:269–73. - PubMed
-
- Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124:394–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

