Celastrol inhibits polyglutamine aggregation and toxicity though induction of the heat shock response
- PMID: 17943263
- PMCID: PMC2262918
- DOI: 10.1007/s00109-007-0251-9
Celastrol inhibits polyglutamine aggregation and toxicity though induction of the heat shock response
Abstract
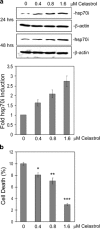
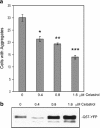
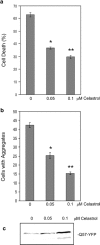
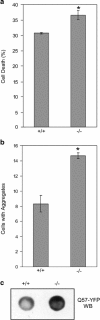
Heat shock proteins (hsps) are protective against the harmful effects of mutant expanded polyglutamine repeat proteins that occur in diseases such as Huntington's, prompting the search for pharmacologic compounds that increase hsp expression in cells as potential treatments for this and related diseases. In this paper, we show that celastrol, a compound recently shown to up-regulate hsp gene expression, significantly decreases killing of cells expressing mutant polyglutamine protein. This effect requires the presence of the transcription factor responsible for mediating inducible hsp gene expression, HSF1, and is correlated with decreased amounts and increased sodium dodecyl sulfate (SDS) solubility of polyglutamine aggregates. These results suggest the potential of celastrol as a therapeutic agent in the treatment of human polyglutamine expansion diseases.
Figures
Similar articles
-
Induction of heat shock proteins in differentiated human neuronal cells following co-application of celastrol and arimoclomol.Cell Stress Chaperones. 2016 Sep;21(5):837-48. doi: 10.1007/s12192-016-0708-2. Epub 2016 Jun 8. Cell Stress Chaperones. 2016. PMID: 27273088 Free PMC article.
-
Dynamic regulation and involvement of the heat shock transcriptional response in arsenic carcinogenesis.J Cell Physiol. 2006 May;207(2):562-9. doi: 10.1002/jcp.20599. J Cell Physiol. 2006. PMID: 16447264
-
Celastrol can inhibit proteasome activity and upregulate the expression of heat shock protein genes, hsp30 and hsp70, in Xenopus laevis A6 cells.Comp Biochem Physiol A Mol Integr Physiol. 2010 Jun;156(2):285-93. doi: 10.1016/j.cbpa.2010.02.015. Epub 2010 Feb 24. Comp Biochem Physiol A Mol Integr Physiol. 2010. PMID: 20188206
-
Celastrol protects ischaemic myocardium through a heat shock response with up-regulation of haeme oxygenase-1.Br J Pharmacol. 2014 Dec;171(23):5265-79. doi: 10.1111/bph.12838. Br J Pharmacol. 2014. PMID: 25041185 Free PMC article.
-
Protective role of HSF1 and HSP70 against gastrointestinal diseases.Int J Hyperthermia. 2009 Dec;25(8):668-76. doi: 10.3109/02656730903213366. Int J Hyperthermia. 2009. Retraction in: Curr Opin Pharmacol. 2014 Dec;19:1-5. doi: 10.1016/j.coph.2014.05.009. PMID: 20021227 Retracted. Review.
Cited by
-
Induction of heat shock proteins in differentiated human neuronal cells following co-application of celastrol and arimoclomol.Cell Stress Chaperones. 2016 Sep;21(5):837-48. doi: 10.1007/s12192-016-0708-2. Epub 2016 Jun 8. Cell Stress Chaperones. 2016. PMID: 27273088 Free PMC article.
-
Plant-derived triterpenoids and analogues as antitumor and anti-HIV agents.Nat Prod Rep. 2009 Oct;26(10):1321-44. doi: 10.1039/b810774m. Epub 2009 Aug 13. Nat Prod Rep. 2009. PMID: 19779642 Free PMC article. Review.
-
Association of heat-shock proteins in various neurodegenerative disorders: is it a master key to open the therapeutic door?Mol Cell Biochem. 2014 Jan;386(1-2):45-61. doi: 10.1007/s11010-013-1844-y. Epub 2013 Oct 5. Mol Cell Biochem. 2014. PMID: 24096700 Review.
-
Hsp70 inhibits aminoglycoside-induced hearing loss and cochlear hair cell death.Cell Stress Chaperones. 2009 Jul;14(4):427-37. doi: 10.1007/s12192-008-0097-2. Epub 2009 Jan 15. Cell Stress Chaperones. 2009. PMID: 19145477 Free PMC article.
-
Protein homeostasis in models of aging and age-related conformational disease.Adv Exp Med Biol. 2010;694:138-59. doi: 10.1007/978-1-4419-7002-2_11. Adv Exp Med Biol. 2010. PMID: 20886762 Free PMC article. Review.
References
-
- Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/S0092-8674(00)80513-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

