Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome
- PMID: 17943779
- PMCID: PMC8554819
- DOI: 10.1002/14651858.CD003063.pub3
Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome
Abstract
Background: Both prophylactic and early surfactant replacement therapy reduce mortality and pulmonary complications in ventilated infants with respiratory distress syndrome (RDS) compared with later selective surfactant administration. However, continued post-surfactant intubation and ventilation are risk factors for bronchopulmonary dysplasia (BPD). The purpose of this review was to compare outcomes between two strategies of surfactant administration in infants with RDS; prophylactic or early surfactant administration followed by prompt extubation, compared with later, selective use of surfactant followed by continued mechanical ventilation.
Objectives: To compare two treatment strategies in preterm infants with or at risk for RDS: early surfactant administration with brief mechanical ventilation (less than one hour) followed by extubation vs. later selective surfactant administration, continued mechanical ventilation, and extubation from low respiratory support. Two populations of infants receiving early surfactant were considered: spontaneously breathing infants with signs of RDS (who receive surfactant administration during evolution of RDS prior to requiring intubation for respiratory failure) and infants at high risk for RDS (who receive prophylactic surfactant administration within 15 minutes after birth).
Search strategy: Searches were made of the Oxford Database of Perinatal Trials, MEDLINE (1966 - December 2006), CINAHL (1982 to December Week 2, 2006), EMBASE (1980 - December 2006), Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 4, 2006), Pediatric Research (1990 - 2006), abstracts, expert informants and hand searching. No language restrictions were applied.
Selection criteria: Randomized or quasi-randomized controlled clinical trials comparing early surfactant administration with planned brief mechanical ventilation (less than one hour) followed by extubation vs. selective surfactant administration continued mechanical ventilation, and extubation from low respiratory support.
Data collection and analysis: Data were sought regarding effects on the incidence of mechanical ventilation (ventilation continued or initiated beyond one hour after surfactant administration), incidence of bronchopulmonary dysplasia (BPD), chronic lung disease (CLD), mortality, duration of mechanical ventilation, duration of hospitalization, duration of oxygen therapy, duration of respiratory support (including CPAP and nasal cannula), number of patients receiving surfactant, number of surfactant doses administered per patient, incidence of air leak syndromes (pulmonary interstitial emphysema, pneumothorax), patent ductus arteriosus requiring treatment, pulmonary hemorrhage, and other complications of prematurity. Stratified analysis was performed according to inspired oxygen threshold for early intubation and surfactant administration in the treatment group: inspired oxygen within lower (FiO2< 0.45) or higher (FiO2 > 0.45) range at study entry. Treatment effect was expressed as relative risk (RR) and risk difference (RD) for categorical variables, and weighted mean difference (WMD) for continuous variables.
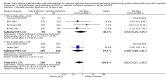
Main results: Six randomized controlled clinical trials met selection criteria and were included in this review. In these studies of infants with signs and symptoms of RDS, intubation and early surfactant therapy followed by extubation to nasal CPAP (NCPAP) compared with later selective surfactant administration was associated with a lower incidence of mechanical ventilation [typical RR 0.67, 95% CI 0.57, 0.79], air leak syndromes [typical RR 0.52, 95% CI 0.28, 0.96] and BPD [typical RR 0.51, 95% CI 0.26, 0.99]. A larger proportion of infants in the early surfactant group received surfactant than in the selective surfactant group [typical RR 1.62, 95% CI 1.41, 1.86]. The number of surfactant doses per patient was significantly greater among patients randomized to the early surfactant group [WMD 0.57 doses per patient, 95% CI 0.44, 0.69]. In stratified analysis by FIO2 at study entry, a lower threshold for treatment (FIO2< 0.45) resulted in lower incidence of airleak [typical RR 0.46 and 95% CI 0.23, 0.93] and BPD [typical RR 0.43, 95% CI 0.20, 0.92]. A higher treatment threshold (FIO2 > 0.45) at study entry was associated with a higher incidence of patent ductus arteriosus requiring treatment [typical RR 2.15, 95% CI 1.09, 4.13].
Authors' conclusions: Early surfactant replacement therapy with extubation to NCPAP compared with later selective surfactant replacement and continued mechanical ventilation with extubation from low ventilator support is associated with less need mechanical ventilation, lower incidence of BPD and fewer air leak syndromes. A lower treatment threshold (FIO2< 0.45) confers greater advantage in reducing the incidences of airleak syndromes and BPD; moreover a higher treatment threshold (FIO2 at study > 0.45) was associated with increased risk of PDA. These data suggest that treatment with surfactant by transient intubation using a low treatment threshold (FIO2< 0.45) is preferable to later, selective surfactant therapy by transient intubation using a higher threshold for study entry (FIO2 > 0.45) or at the time of respiratory failure and initiation of mechanical ventilation.
Conflict of interest statement
Dr. R. Soll is the principal investigator for several trials of pulmonary surfactant and has acted as a paid consultant for several of the pharmaceutical companies that manufacture surfactant products (Abbott Laboratories, Dey Laboratories, Ross Laboratories).
Figures













Update of
-
Early surfactant administration with brief ventilation vs selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome.Cochrane Database Syst Rev. 2004;(3):CD003063. doi: 10.1002/14651858.CD003063.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2007 Oct 17;(4):CD003063. doi: 10.1002/14651858.CD003063.pub3. PMID: 15266470 Updated.
References
References to studies included in this review
Dani 2004 {published data only}
-
- Dani C, Bertini G, Pezzati M, Cecchi A, Caviglioli C, Rubaltelli FF. Early extubation and nasal continuous positive airway pressure after surfactant treatment in preterm infants of less than 30 weeks' gestation. Pediatrics 2004;113:e560‐3. - PubMed
NICHD 2002 {published and unpublished data}
-
- Haberman B, Shankaran S, Stevenson DK, Papile LA, Stark A, Korones S, et al. Does surfactant and immediate extubation to nasal continuous positive airway pressure reduce use of mechanical ventilation?. Pediatric Research 2002;51:349A.
Reininger 2005 {published data only}
-
- Reininger A, Khalak R, Kendig JW, Ryan RM, Stevens TP, Reubens L, D'Angio CT. Surfactant administration by transient intubation in infants 29 to 35 weeks' gestation with respiratory distress syndrome decreases need of later mechanical ventilation: a randomized controlled trial. Journal of Perinatology 2005;25:703‐8. - PubMed
Texas Research 2004 {published data only}
-
- The Texas Neonatal Research Group, 2004. Early surfactant for neonates with mild to moderate respiratory distress syndrome: A multicenter randomized trial. Journal of Pediatrics 2004;144:804‐8. - PubMed
Verder 1994 {published data only}
-
- Verder H, Robertson B, Greisen G, Ebbesen F, Albertsen P, Lundstrom K, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. The New England Journal of Medicine 1994;331:1051‐5. - PubMed
Vermont Oxford 2003 {published and unpublished data}
-
- Soll RF, Conner JM, Howard D and the Investigators of the Early Surfactant Replacement Study. Early surfactant replacement in spontaneously breathing premature infants with RDS. Pediatric Research 2003:Late Breaker Abstract 12, PAS 2003 meeting.
References to studies excluded from this review
Alba 1995 {published data only}
-
- Alba J, Agarwal R, Hegyi T, Hiatt IM. Efficacy of surfactant therapy in infants managed with CPAP. Pediatric Pulmonology 1995;20:172‐6. - PubMed
Blennow 1999 {published data only}
-
- Blennow M, Jonsson B, Dahlstrom A, Sarman I, Bohlin K, Robertson B. [Lung function in premature infants can be improved. Surfactant therapy and CPAP reduce the need of respiratory support]. [Swedish]. Lakartidningen 1999;96:1571‐6. - PubMed
Dambeanu 1997 {published data only}
-
- Dambeanu JM, Parmigiani S, Marinescu B, Bevilacqua G. Use of surfactant for prevention of respiratory distress syndrome in newborn infants in spontaneous breathing. A randomized multicentre clinical pilot‐study. Acta Bio‐medica de L'Ateneo Parmense 1997;68 Suppl 1:39‐45. - PubMed
Lefort 2003 {published data only}
-
- Lefort S, Diniz EM, Vaz FA. Clinical course of premature infants intubated in the delivery room, submitted or not to porcine‐derived lung surfactant therapy within the first hour of life. Journal of Maternal‐Fetal and Neonatal Medicine 2003;14:187‐96. - PubMed
Mandy 1998 {published data only}
-
- Mandy GT, Moise AA, Smith EO, Hansen TN. Endotracheal continuous positive airway pressure after rescue surfactant therapy. Journal of Perinatology 1998;18:444‐8. - PubMed
Sandri 2004 {published data only}
-
- Sandri F, Ancora G, Lanzoni A, Tagliabue P, Colnaghi M, Ventura ML, et al. Prophylactic nasal continuous postive airways pressure in newborns of 28‐31 weeks' gestation: multicentre randomised controlled clinical trial. Archives of Disease in Childhood Fetal and Neonatal Edition 2004;89:F394‐8. - PMC - PubMed
So 1994 {published data only}
-
- So BH, Tamura M, Kamoshita S. Nasal continuous positive airway pressure following surfactant replacement for the treatment of neonatal respiratory distress syndrome. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1994;35:280‐7. - PubMed
Tooley 2003 {published data only}
-
- Tooley J, Dyke M. Randomized study of nasal continuous positive airway pressure in the preterm infant with respiratory distress syndrome. Acta Paediatrica 2003;92:1170‐4. - PubMed
Verder 1992 {published data only}
-
- Verder H, Agertoft L, Albertsen P, Christensen NC, Curstedt T, Ebbesen F, et al. [Surfactant treatment of newborn infants with respiratory distress syndrome primarily treated with nasal continuous positive air pressure. A pilot study]. Ugeskrift for Laeger 1992;154:2136‐9. - PubMed
Verder 1999 {published data only}
-
- Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A, et al. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks' gestation. Pediatrics 1999;103:E24. - PubMed
Victorin 1990 {published data only}
-
- Victorin LH, Deverajan LV, Curstedt T, Robertson B. Surfactant replacement in spontaneously breathing babies with hyaline membrane disease ‐ a pilot study. Biology of the Neonate 1990;58:121‐6. - PubMed
References to studies awaiting assessment
Thomson 2002 {published data only}
-
- Thomson MA. Continuous positive airway pressure and surfactant; combined data from animal experiments and clinical trials. Biology of the Neonate 2002;81:16‐9. - PubMed
Additional references
Avery 1987
-
- Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers.. Pediatrics 1987;79:26‐30. - PubMed
D'Angio 2003
-
- D'Angio CT, Khalak R, Stevens TP, Reininger A, Reubens L, Kendig JW, Ryan RM. Intratracheal surfactant administration by transient intubation in infants 29‐35 weeks' gestation with RDS requiring nasal CPAP decreases the likelihood of later mechanical ventilation: A randomized controlled trial. Pediatric Research 2003;53:367A. - PubMed
Gortner 1998
-
- Gortner L, Wauer RR, Hammer H, Stock GJ, Heitmann F, Reiter HL, et al. Early versus late surfactant treatment in preterm infants of 27 to 32 weeks' gestational age: a multicenter controlled clinical trial. Pediatrics 1998;102:1153‐60. - PubMed
Greenough 2002
Gregory 1971
-
- Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory‐distress syndrome with continuous positive airway pressure. New England Journal of Medicine 1971;284:1333‐40. - PubMed
Ho 2002
-
- Ho JJ, Subramaniam P, Henderson‐Smart DJ. Continuous distending pressure for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD002271] - PubMed
Jonsson 1997
-
- Jonsson B, Katz‐Salamon M, Faxelius G, Broberger U, Lagercrantz H. Neonatal care of very‐low‐birthweight infants in special‐care units and neonatal intensive‐care units in Stockholm. Early nasal continuous positive airway pressure versus mechanical ventilation: gains and losses. Acta Paediatrica 1997;419 (Suppl Apr):4‐10. - PubMed
Kamper 1999
-
- Kamper J. Early nasal continous positive airway pressure and minimal handling in the treatment of very‐low‐birthweight infants. Biology of the Neonate 1999;76(Suppl 1):22‐8. - PubMed
Northway 1967
-
- Northway WH, Rosan RC, Parker DY. Pulmonary disease following respiratory therapy of hyaline membrane disease. The New England Journal of Medicine 1967;276:357‐74. - PubMed
Soll 2002a
Soll 2002b
Van Marter 2000
-
- Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, Susser M, Paneth N, Leviton A. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics 2000;105:1194‐1201. - PubMed
References to other published versions of this review
Stevens 2002
-
- Stevens TP, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs selective surfactant and continued mechanical ventilation for preterm infants with or at risk for RDS. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD003063.pub2] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

