Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
- PMID: 17943917
- PMCID: PMC4069257
- DOI: 10.1002/14651858.CD006826
Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease
Update in
-
Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2013 Aug 30;2013(8):CD006826. doi: 10.1002/14651858.CD006826.pub2. Cochrane Database Syst Rev. 2013. PMID: 23990350 Free PMC article.
Abstract
Background: Long-acting beta-agonists and inhaled corticosteroids have both been recommended in guidelines for the treatment of chronic obstructive pulmonary disease. Their co-administration in a combined inhaler is intended to facilitate adherence to medication regimens, and to improve efficacy. Two preparations are currently available, fluticasone/salmeterol (FPS) and budesonide/formoterol (BDF).
Objectives: To assess the efficacy of combined inhaled corticosteroid and long-acting beta-agonist preparations, compared to inhaled corticosteroids, in the treatment of adults with chronic obstructive pulmonary disease.
Search strategy: We searched the Cochrane Airways Group Specialised Register of trials. The date of the most recent search is April 2007.
Selection criteria: Studies were included if they were randomised and double-blind. Studies compared combined inhaled corticosteroids and long-acting beta-agonist preparations with the inhaled corticosteroid component.
Data collection and analysis: Two reviewers independently assessed trial quality and extracted data. The primary outcome were exacerbations, mortality and pneumonia. Health-related quality of life (measured by validated scales), lung function and side-effects were secondary outcomes. Dichotomous data were analysed as fixed effect odds ratios (OR), and continuous data as mean differences and 95% confidence intervals (CI).
Main results: Seven studies of good methodological quality met the inclusion criteria randomising 5708 participants with predominantly poorly reversible, severe COPD. Exacerbation rates were significantly reduced with combination therapies (Rate ratio 0.91; 95% confidence interval 0.85 to 0.97, P = 0.0008). Data from two FPS studies indicated that exacerbations requiring oral steroids were reduced with combination therapy. Data from one large study suggest that there is no significant difference in the rate of hospitalisations. Mortality was also lower with combined treatment (odds ratio 0.77; 95% confidence interval 0.63 to 0.94). Quality of life, lung function and withdrawals due to lack of efficacy favoured combination treatment. Adverse event profiles were similar between the two treatments. No significant differences were found between FPS and BDP in the primary outcomes, but the confidence intervals for the BDP results were wide as smaller numbers of patients have been studied.
Authors' conclusions: Combination ICS and LABA significantly reduces morbidity and mortality in COPD when compared with mono component steroid. Adverse events were not significantly different between treatments, although evidence from other sources indicates that inhaled corticosteroids are associated with increased risk of pneumonia. Assessment of BDF in larger, long-term trials is required. Dose response data would provide valuable evidence on whether efficacy and safety outcomes are affected by different steroid loads.
Figures

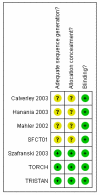



Comment in
-
Review: corticosteroids plus LABAs reduce exacerbations and mortality more than steroids alone in COPD.Evid Based Med. 2008 Jun;13(3):74. doi: 10.1136/ebm.13.3.74. Evid Based Med. 2008. PMID: 18515621 No abstract available.
References
References to studies included in this review
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]; American Thoracic Society 100th International Conference; 2004; May 21-26, p. C22. Poster 505.
-
-
*
- Calverley PM, Bonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal. 2003;22(6):912–9. - PubMed
-
-
- Calverley PMA, Cseke Z, Peterson S. Budesonide/formoterol reduces the use of oral corticosteroids in the treatment of COPD [Abstract] European Respiratory Journal. 2003;22(Suppl 45):P436. - PubMed
-
- Calverley PMA, Kuna P, Olsson H. COPD exacerbations are reduced by budesonide/formoterol in a single inhaler [Abstract] European Respiratory Journal. 2003;22(Suppl 45):P1587.
-
- Calverley PMA, Olsson H, Symbicort International COPD Study Group Budesonide/formoterol ina single inhaler sustains improvements in lung function over 12 months compared with monocomponents and placebo in patients with COPD [abstract]; American Thoracic Society 99th International Conference; 2003; p. B024. Poster 418.
References to studies awaiting assessment
-
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine. 2007;146(8):545–55. - PubMed
-
- Cukier A, Ferreira CAS, Stelmach R, Ribeiro M, Cortopassi F, Calverley PMA. The effect of bronchodilators and oxygen alone and in combination on self-paced exercise performance in stable COPD. Respiratory Medicine. 2007;101(4):743–53. - PubMed
-
- Golabi P, Topaloglu N, Karakurt S, Celikel T. Effects of tiotropium and salmeterol/fluticasone combination on lung hyperinflation dyspnea and exercise tolerance in COPD [Abstract] European Respiratory Journal. 2006;28(Suppl 50):33s.
Additional references
-
- American Thoracic Society Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(5 Pt 2):S77–121. - PubMed
-
- Global Strategy for Diagnosis, Management, Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2006. http://www.goldcopd.org.
-
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. European Respiratory Journal. 2002;19:398–404. - PubMed
References to other published versions of this review
-
- Nannini L, Lasserson TJ, Poole P. Combined corticosteroid and longacting beta-agonist in one inhaler for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2003;(4) CD003794. - PubMed
-
- Nannini L, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and longacting beta-agonist in one inhaler for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2004;(3):CD003794. - PubMed
-
-
* Indicates the major publication for the study
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

