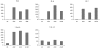LUD 00-009: phase 1 study of intensive course immunization with NY-ESO-1 peptides in HLA-A2 positive patients with NY-ESO-1-expressing cancer
- PMID: 17944437
- PMCID: PMC2935748
LUD 00-009: phase 1 study of intensive course immunization with NY-ESO-1 peptides in HLA-A2 positive patients with NY-ESO-1-expressing cancer
Abstract
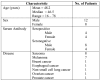
NY-ESO-1 is a cancer-testis antigen and an attractive target for immunotherapy in patients with different malignancies. Here we report the results of a phase I clinical study of intensive course NY-ESO-1 peptide vaccination, evaluating the safety, immunogenicity and clinical response in HLA-A2 positive patients with NY-ESO-1 expressing cancers. Of 20 patients enrolled in the trial, 14 completed at least 2 cycles of immunization and were evaluable for clinical and immunological response. Five of these evaluable patients were treated in cohort 1 (baseline seropositive) and 9 patients were treated in cohort 2 (baseline seronegative). During vaccination, NY-ESO-1-specific CD8+ T-cells were induced in 3 of 9 baseline seronegative patients. In patients with pre-existing antigen-specific CD8+ T-cells, their number increased or remained stable. In contrast to previous immunization protocols with less intensive immunization schedules, we observed a rapid induction of high magnitude NY-ESO-1 peptide-specific T-cell responses detectable already on day 15-22 of immunization. A specific immune response of high magnitude and early onset may be more effective in eliminating minimal residual disease in adjuvant treatment situations and in preventing tumor progression due to immune escape mechanisms.
Figures
References
-
- Gnjatic S, Atanackovic D, Jäger E, Matsuo M, Selvakumar A, Altorki NK, Maki RG, Dupont B, Ritter G, Chen YT, Knuth A, Old LJ. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: correlation with antibody responses. Proc Natl Acad Sci U S A. 2003;100:8862–8867. - PMC - PubMed
-
- Jäger E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jäger D, Arand M, Ritter G, Old LJ, Knuth A. Humoral immune responses of cancer patients against "Cancer-Testis" antigen NY-ESO-1: correlation with clinical events. Int J Cancer. 1999;84:506–510. - PubMed
-
- Sugita Y, Wada H, Fujita S, Nakata T, Sato S, Noguchi Y, Jungbluth AA, Yamaguchi M, Chen YT, Stockert E, Gnjatic S, Williamson B, Scanlan MJ, Ono T, Sakita I, Yasui M, Miyoshi Y, Tamaki Y, Matsuura N, Noguchi S, Old LJ, Nakayama E, Monden M. NY-ESO-1 expression and immunogenicity in malignant and benign breast tumors. Cancer Res. 2004;64:2199–2204. - PubMed
-
- Valmori D, Dutoit V, Lienard D, Rimoldi D, Pittet MJ, Champagne P, Ellefsen K, Sahin U, Speiser D, Lejeune F, Cerottini JC, Romero P. Naturally occurring human lymphocyte antigen-A2 restricted CD8+ T-cell response to the cancer testis antigen NY-ESO-1 in melanoma patients. Cancer Res. 2000;60:4499–4506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials