Synthesis of the C1-C52 fragment of amphidinol 3, featuring a beta-alkoxy alkyllithium addition reaction
- PMID: 17944478
- PMCID: PMC2496920
- DOI: 10.1021/ol7020934
Synthesis of the C1-C52 fragment of amphidinol 3, featuring a beta-alkoxy alkyllithium addition reaction
Abstract
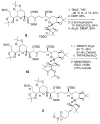
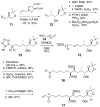
An advanced intermediate for the synthesis of amphidinol 3 has been prepared. A cross-metathesis reaction was used to couple the C1-C12 and C13-C26 segments. An unusual beta-alkoxy alkyllithium reagent was generated from this segment and added to a Weinreb amide to assemble the C1-C52 section of amphidinol 3. These compounds represent some of the most advanced intermediates reported to date for the synthesis of amphidinol 3.
Figures
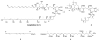
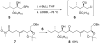

Similar articles
-
Diastereoselective synthesis of the C17-C30 fragment of amphidinol 3.Org Biomol Chem. 2012 Dec 21;10(47):9418-28. doi: 10.1039/c2ob26641e. Epub 2012 Oct 30. Org Biomol Chem. 2012. PMID: 23108742
-
Stereoselective synthesis of the C1-C29 part of amphidinol 3.J Org Chem. 2015 Jan 16;80(2):859-71. doi: 10.1021/jo502322m. Epub 2015 Jan 7. J Org Chem. 2015. PMID: 25517178
-
A C-glycosidation approach to the central core of amphidinol 3: synthesis of the c39-c52 fragment.Org Lett. 2005 Apr 28;7(9):1853-6. doi: 10.1021/ol050477l. Org Lett. 2005. PMID: 15844923
-
Recent applications in natural product synthesis of dihydrofuran and -pyran formation by ring-closing alkene metathesis.Org Biomol Chem. 2016 Jul 7;14(25):5875-93. doi: 10.1039/c6ob00593d. Epub 2016 Apr 25. Org Biomol Chem. 2016. PMID: 27108941 Review.
-
Isolation, structural determination and synthetic approaches toward amphidinol 3.Nat Prod Rep. 2014 Apr;31(4):468-88. doi: 10.1039/c3np70062c. Epub 2014 Feb 17. Nat Prod Rep. 2014. PMID: 24531818 Review.
Cited by
-
Chemoselective synthesis of ketones and ketimines by addition of organometallic reagents to secondary amides.Nat Chem. 2012 Feb 21;4(3):228-34. doi: 10.1038/nchem.1268. Nat Chem. 2012. PMID: 22354438
-
Karlotoxin synthetic studies: concise synthesis of a C(42-63) B-ring tetrahydropyran fragment.Tetrahedron Lett. 2013 Nov 1;54(48):10.1016/j.tetlet.2013.09.104. doi: 10.1016/j.tetlet.2013.09.104. Tetrahedron Lett. 2013. PMID: 24376284 Free PMC article.
-
A highly convergent synthesis of the C1-C31 polyol domain of amphidinol 3 featuring a TST-RCM reaction: confirmation of the revised relative stereochemistry.Chem Sci. 2015 Nov 1;6(11):6407-6412. doi: 10.1039/c5sc00814j. Epub 2015 Aug 6. Chem Sci. 2015. PMID: 28757956 Free PMC article.
-
Enantioselective, Lewis Base-Catalyzed, Intermolecular Sulfenoamination of Alkenes.J Am Chem Soc. 2019 Sep 4;141(35):13767-13771. doi: 10.1021/jacs.9b07019. Epub 2019 Aug 21. J Am Chem Soc. 2019. PMID: 31433174 Free PMC article.
-
INTEGRATED APPROACHES TO THE CONFIGURATIONAL ASSIGNMENT OF MARINE NATURAL PRODUCTS.Tetrahedron. 2012 Nov 18;68(46):9307-9343. doi: 10.1016/j.tet.2011.12.070. Tetrahedron. 2012. PMID: 23814320 Free PMC article. No abstract available.
References
-
- Murata M, Matsuoka S, Matsumori N, Paul GK, Tachibana K. J Am Chem Soc. 1999;121:870–871.
-
- Paul GK, Matsumori N, Murata M, Tachibana K. Tetrahedron Lett. 1995;36:6279–6282.
- Satake M, Murata M, Yasumoto T, Fujita T, Naoki H. J Am Chem Soc. 1991;113:9859–9861.
- Houdai T, Matsuoka S, Murata M, Satake M, Ota S, Oshima Y, Rhodes LL. Tetrahedron. 2001;57:5551–5555.
- Paul GK, Matsumori N, Konoki K, Murata M, Tachibana K. J Mar Biotechnol. 1997;5:124–128.
- Echigoya R, Rhodes L, Oshima Y, Satake M. Harmful Algae. 2005;4:383–389.
- Doi Y, Ishibshi M, Nakamichi H, Kosaka T, Ishikawa T, Kobayashi Ji. J Org Chem. 1997;62:3820–3823.
- Huang XC, Zhao D, Guo YW, Wu HM, Trivellone E, Cimino G. Tetrahedron Lett. 2004;45:5501–5504.
-
- Matsumori N, Kaneno D, Murata M, Nakamura H, Tachibana K. J Org Chem. 1999;64:866–876. - PubMed
-
- BouzBouz S, Cossy J. Org Lett. 2001;3:1451–1454. - PubMed
- Flamme EM, Roush WR. Org Lett. 2005;7:1411–1414. - PubMed
- Hicks JD, Flamme EM, Roush WR. Org Lett. 2005;7:5509–5512. - PubMed
- Chang SK, Paquette LA. Synlett. 2005:2915–2918.
- Paquette LA, Chang SK. Org Lett. 2005;7:3111–3114. - PubMed
- Dubost C, Marko IE, Bryans J. Tetrahedron Lett. 2005;46:4005–4009.
- Bedore MW, Chang SK, Paquette LA. Org Lett. 2007;9:513–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

