Beta7-integrin-independent enhancement of mucosal and systemic anti-HIV antibody responses following combined mucosal and systemic gene delivery
- PMID: 17944930
- PMCID: PMC2433338
- DOI: 10.1111/j.1365-2567.2007.02702.x
Beta7-integrin-independent enhancement of mucosal and systemic anti-HIV antibody responses following combined mucosal and systemic gene delivery
Abstract
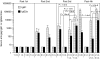
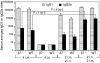
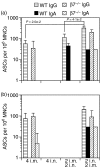
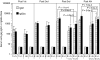
Vaccination strategies that can block or limit heterosexual human immunodeficiency virus (HIV) transmissions to local and systemic tissues are the goal of much research effort. Herein, in a mouse model, we aimed to determine whether the enhancement of antibody responses through mucosal and systemic immunizations, previously observed with protein-based vaccines, applies to immunizations with DNA- or RNA-based vectors. Intranasal (i.n.) followed by intramuscular (i.m.) immunizations (i.n./i.m.) with polylactide-coglycolide (PLG)-DNA microparticles encoding HIV-gag (PLG-DNA-gag) significantly enhanced serum antibody responses, compared with i.m., i.n. or i.m. followed by i.n. (i.m./i.n.) immunizations. Moreover, while i.n./i.m., i.n. or i.m./i.n. immunizations with PLG-DNA-gag resulted in genital tract antibody responses, i.m. immunizations alone failed to do so. Importantly, beta7-deficient mice developed local and systemic antibody responses following i.n./i.m. immunization, or immunization via any other route, similar to those of wild-type mice. To compare the DNA with an RNA delivery system, immunizations were performed with VEE/SIN-gag replicon particles, composed of Venezuelan equine encephalitis virus (VEE) replicon RNA and Sindbis surface structure (SIN). i.n./i.m., compared with any other immunizations, i.n./i.m. immunization with VEE/SIN-gag resulted in enhanced genital tract but not serum antibody responses. These data show for the first time that mucosal followed by systemic immunizations with gene delivery systems enhance B-cell responses independent of the mucosal homing receptors alpha4beta7 and alphaEbeta7.
Figures


Similar articles
-
Characterization of human immunodeficiency virus Gag-specific gamma interferon-expressing cells following protective mucosal immunization with alphavirus replicon particles.J Virol. 2005 Jun;79(11):7135-45. doi: 10.1128/JVI.79.11.7135-7145.2005. J Virol. 2005. PMID: 15890953 Free PMC article.
-
Antibody responses against HIV in rhesus macaques following combinations of mucosal and systemic immunizations with chimeric alphavirus-based replicon particles.AIDS Res Hum Retroviruses. 2006 Oct;22(10):993-7. doi: 10.1089/aid.2006.22.993. AIDS Res Hum Retroviruses. 2006. PMID: 17067269
-
Long-term protection in hamsters against human parainfluenza virus type 3 following mucosal or combinations of mucosal and systemic immunizations with chimeric alphavirus-based replicon particles.Scand J Immunol. 2007 Dec;66(6):645-53. doi: 10.1111/j.1365-3083.2007.02019.x. Epub 2007 Oct 17. Scand J Immunol. 2007. PMID: 17944814
-
Human immunodeficiency virus type 1 Gag-specific vaginal immunity and protection after local immunizations with sindbis virus-based replicon particles.J Infect Dis. 2001 Dec 15;184(12):1613-6. doi: 10.1086/324581. Epub 2001 Dec 3. J Infect Dis. 2001. PMID: 11740739
-
Clarification of how HIV-1 DNA and protein immunizations may be better used to obtain HIV-1-specific mucosal and systemic immunity.Expert Rev Vaccines. 2007 Apr;6(2):203-12. doi: 10.1586/14760584.6.2.203. Expert Rev Vaccines. 2007. PMID: 17408370 Review.
Cited by
-
Mucosal immunity and protection against HIV/SIV infection: strategies and challenges for vaccine design.Int Rev Immunol. 2009;28(1):20-48. doi: 10.1080/08830180802684331. Int Rev Immunol. 2009. PMID: 19241252 Free PMC article. Review.
-
Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge.J Virol. 2011 Aug;85(16):8316-27. doi: 10.1128/JVI.00781-11. Epub 2011 Jun 8. J Virol. 2011. PMID: 21653670 Free PMC article.
-
Alphavirus replicon particles acting as adjuvants promote CD8+ T cell responses to co-delivered antigen.Vaccine. 2008 Aug 5;26(33):4267-75. doi: 10.1016/j.vaccine.2008.05.046. Epub 2008 Jun 9. Vaccine. 2008. PMID: 18582997 Free PMC article.
-
Adhesion Molecules Associated with Female Genital Tract Infection.PLoS One. 2016 Jun 7;11(6):e0156605. doi: 10.1371/journal.pone.0156605. eCollection 2016. PLoS One. 2016. PMID: 27272720 Free PMC article.
-
Prolonged protection against Intranasal challenge with influenza virus following systemic immunization or combinations of mucosal and systemic immunizations with a heat-labile toxin mutant.Clin Vaccine Immunol. 2009 Apr;16(4):471-8. doi: 10.1128/CVI.00311-08. Epub 2009 Feb 4. Clin Vaccine Immunol. 2009. PMID: 19193829 Free PMC article.
References
-
- Quinn TC. Global burden of the HIV pandemic. Lancet. 1996;348:99–106. - PubMed
-
- Parr MB, Parr EL. Vaginal immunity in the HSV-2 mouse model. Int Rev Immunol. 2003;22:43–63. - PubMed
-
- Miller CJ, Lu FX. Anti-HIV and -SIV immunity in the vagina. Int Rev Immunol. 2003;22:65–76. - PubMed
-
- Vajdy M. Induction of optimal immune responses against human immunodeficiency virus at mucosal portals of entry. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:222–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

