Common cellular events occur during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima
- PMID: 17945004
- PMCID: PMC2176065
- DOI: 10.1186/1471-213X-7-115
Common cellular events occur during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima
Abstract
Background: All animals possess some type of tissue repair mechanism. In some species, the capacity to repair tissues is limited to the healing of wounds. Other species, such as echinoderms, posses a striking repair capability that can include the replacement of entire organs. It has been reported that some mechanisms, namely extracellular matrix remodeling, appear to occur in most repair processes. However, it remains unclear to what extent the process of organ regeneration, particularly in animals where loss and regeneration of complex structures is a programmed natural event, is similar to wound healing. We have now used the sea cucumber Holothuria glaberrima to address this question.
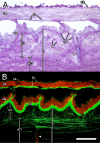
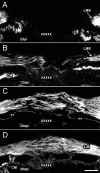
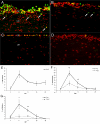
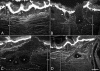
Results: Animals were lesioned by making a 3-5 mm transverse incision between one of the longitudinal muscle pairs along the bodywall. Lesioned tissues included muscle, nerve, water canal and dermis. Animals were allowed to heal for up to four weeks (2, 6, 12, 20, and 28 days post-injury) before sacrificed. Tissues were sectioned in a cryostat and changes in cellular and tissue elements during repair were evaluated using classical dyes, immmuohistochemistry and phalloidin labeling. In addition, the temporal and spatial distribution of cell proliferation in the animals was assayed using BrdU incorporation. We found that cellular events associated with wound healing in H. glaberrima correspond to those previously shown to occur during intestinal regeneration. These include: (1) an increase in the number of spherule-containing cells, (2) remodeling of the extracellular matrix, (3) formation of spindle-like structures that signal dedifferentiation of muscle cells in the area flanking the lesion site and (4) intense cellular division occurring mainly in the coelomic epithelium after the first week of regeneration.
Conclusion: Our data indicate that H. glaberrima employs analogous cellular mechanisms during wound healing and organ regeneration. Thus, it is possible that regenerative limitations in some organisms are due either to the absence of particular mechanisms associated with repair or the inability of activating the repair process in some tissues or stages.
Figures



Similar articles
-
Regeneration of the radial nerve cord in the sea cucumber Holothuria glaberrima.BMC Dev Biol. 2009 Jan 6;9:3. doi: 10.1186/1471-213X-9-3. BMC Dev Biol. 2009. PMID: 19126208 Free PMC article.
-
Spherulocytes in the echinoderm Holothuria glaberrima and their involvement in intestinal regeneration.Dev Dyn. 2006 Dec;235(12):3259-67. doi: 10.1002/dvdy.20983. Dev Dyn. 2006. PMID: 17061269
-
Retinoic Acid Signaling Is Associated with Cell Proliferation, Muscle Cell Dedifferentiation, and Overall Rudiment Size during Intestinal Regeneration in the Sea Cucumber, Holothuria glaberrima.Biomolecules. 2019 Dec 13;9(12):873. doi: 10.3390/biom9120873. Biomolecules. 2019. PMID: 31847189 Free PMC article.
-
Holothurians as a Model System to Study Regeneration.Results Probl Cell Differ. 2018;65:255-283. doi: 10.1007/978-3-319-92486-1_13. Results Probl Cell Differ. 2018. PMID: 30083924 Review.
-
A roadmap for intestinal regeneration.Int J Dev Biol. 2021;65(4-5-6):427-437. doi: 10.1387/ijdb.200227dq. Int J Dev Biol. 2021. PMID: 32930367 Free PMC article. Review.
Cited by
-
Radial glial cells play a key role in echinoderm neural regeneration.BMC Biol. 2013 Apr 18;11:49. doi: 10.1186/1741-7007-11-49. BMC Biol. 2013. PMID: 23597108 Free PMC article.
-
Identification and expression of the elongator protein 2 (Ajelp2) gene, a novel regeneration-related gene from the sea cucumber Apostichopus japonicus.Mol Biol Rep. 2014 Aug;41(8):4985-96. doi: 10.1007/s11033-014-3365-5. Epub 2014 Apr 20. Mol Biol Rep. 2014. PMID: 24748431
-
Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector.Mar Drugs. 2020 May 22;18(5):274. doi: 10.3390/md18050274. Mar Drugs. 2020. PMID: 32455954 Free PMC article. Review.
-
Proliferative Effect of Aqueous Extract of Sea Cucumber (Holothuria parva) Body Wall on Human Umbilical Cord Mesenchymal Stromal/Stem Cells.Mar Drugs. 2023 Apr 26;21(5):267. doi: 10.3390/md21050267. Mar Drugs. 2023. PMID: 37233461 Free PMC article.
-
Characterization of Two Novel EF-Hand Proteins Identifies a Clade of Putative Ca2+-Binding Protein Specific to the Ambulacraria.J Bioinform Syst Biol. 2022;5(1):1-25. doi: 10.26502/jbsb.5107030. Epub 2022 Feb 3. J Bioinform Syst Biol. 2022. PMID: 36382242 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

