Subunit conformations and assembly states of a DNA-translocating motor: the terminase of bacteriophage P22
- PMID: 17945256
- PMCID: PMC2204089
- DOI: 10.1016/j.jmb.2007.08.070
Subunit conformations and assembly states of a DNA-translocating motor: the terminase of bacteriophage P22
Abstract
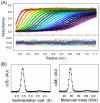
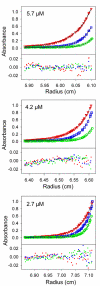
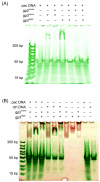
Bacteriophage P22, a podovirus infecting strains of Salmonella typhimurium, packages a 42-kbp genome using a headful mechanism. DNA translocation is accomplished by the phage terminase, a powerful molecular motor consisting of large and small subunits. Although many of the structural proteins of the P22 virion have been well characterized, little is known about the terminase subunits and their molecular mechanism of DNA translocation. We report here structural and assembly properties of ectopically expressed and highly purified terminase large and small subunits. The large subunit (gp2), which contains the nuclease and ATPase activities of terminase, exists as a stable monomer with an alpha/beta fold. The small subunit (gp3), which recognizes DNA for packaging and may regulate gp2 activity, exhibits a highly alpha-helical secondary structure and self-associates to form a stable oligomeric ring in solution. For wild-type gp3, the ring contains nine subunits, as demonstrated by hydrodynamic measurements, electron microscopy, and native mass spectrometry. We have also characterized a gp3 mutant (Ala 112-->Thr) that forms a 10-subunit ring, despite a subunit fold indistinguishable from wild type. Both the nonameric and decameric gp3 rings exhibit nonspecific DNA-binding activity, and gp2 is able to bind strongly to the DNA/gp3 complex but not to DNA alone. We propose a scheme for the roles of P22 terminase large and small subunits in the recruitment and packaging of viral DNA and discuss the model in relation to proposals for terminase-driven DNA translocation in other phages.
Figures







Similar articles
-
Variable Assembly and Procapsid Binding of Bacteriophage P22 Terminase Subunits in Solution.Pathogens. 2024 Dec 3;13(12):1066. doi: 10.3390/pathogens13121066. Pathogens. 2024. PMID: 39770326 Free PMC article.
-
Assembly architecture and DNA binding of the bacteriophage P22 terminase small subunit.J Mol Biol. 2008 Nov 14;383(3):494-501. doi: 10.1016/j.jmb.2008.08.050. Epub 2008 Aug 27. J Mol Biol. 2008. PMID: 18775728 Free PMC article.
-
Architecture of the Complex Formed by Large and Small Terminase Subunits from Bacteriophage P22.J Mol Biol. 2015 Oct 9;427(20):3285-3299. doi: 10.1016/j.jmb.2015.08.013. Epub 2015 Aug 21. J Mol Biol. 2015. PMID: 26301600 Free PMC article.
-
Structure, assembly, and DNA packaging of the bacteriophage T4 head.Adv Virus Res. 2012;82:119-53. doi: 10.1016/B978-0-12-394621-8.00018-2. Adv Virus Res. 2012. PMID: 22420853 Free PMC article. Review.
-
The bacteriophage DNA packaging machine.Adv Exp Med Biol. 2012;726:489-509. doi: 10.1007/978-1-4614-0980-9_22. Adv Exp Med Biol. 2012. PMID: 22297528 Review.
Cited by
-
Small terminase couples viral DNA binding to genome-packaging ATPase activity.Structure. 2012 Aug 8;20(8):1403-13. doi: 10.1016/j.str.2012.05.014. Epub 2012 Jul 5. Structure. 2012. PMID: 22771211 Free PMC article.
-
Breaking Symmetry in Viral Icosahedral Capsids as Seen through the Lenses of X-ray Crystallography and Cryo-Electron Microscopy.Viruses. 2018 Feb 7;10(2):67. doi: 10.3390/v10020067. Viruses. 2018. PMID: 29414851 Free PMC article. Review.
-
Structure of p22 headful packaging nuclease.J Biol Chem. 2012 Aug 10;287(33):28196-205. doi: 10.1074/jbc.M112.349894. Epub 2012 Jun 19. J Biol Chem. 2012. PMID: 22715098 Free PMC article.
-
Bacteriophage protein-protein interactions.Adv Virus Res. 2012;83:219-98. doi: 10.1016/B978-0-12-394438-2.00006-2. Adv Virus Res. 2012. PMID: 22748812 Free PMC article. Review.
-
Structure and inhibition of herpesvirus DNA packaging terminase nuclease domain.Proc Natl Acad Sci U S A. 2010 Sep 14;107(37):16078-83. doi: 10.1073/pnas.1007144107. Epub 2010 Aug 30. Proc Natl Acad Sci U S A. 2010. PMID: 20805464 Free PMC article.
References
-
- Catalano CE. Viral Genome Packaging Machines: Genetics, Structure, and Mechanism. Landes Bioscience / Eurekah.com and Kluwer Academic / Plenum Publishers; Georgetown, TX and New York: 2005.
-
- Brown JC, McVoy MA, Homa FL. Packaging DNA into Herpesvirus Capsids. In: Holzenburg A, Bogner E, editors. Structure-Function Relationships of Human Pathogenic Viruses. Springer US; Cambridge, MA: 2002. pp. 111–153.
-
- Casjens S, Hayden M. Analysis in vivo of the bacteriophage P22 headful nuclease. J. Mol. Biol. 1988;199:467–474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

