The Epstein-Barr virus BMRF-2 protein facilitates virus attachment to oral epithelial cells
- PMID: 17945327
- PMCID: PMC2268893
- DOI: 10.1016/j.virol.2007.09.012
The Epstein-Barr virus BMRF-2 protein facilitates virus attachment to oral epithelial cells
Abstract
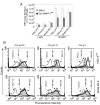
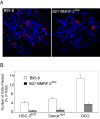
We previously reported that BMRF-2, an Epstein-Barr virus (EBV) glycoprotein, binds to beta1 family integrins and is important for EBV infection of polarized oral epithelial cells. To further study the functions of BMRF-2, we constructed a recombinant EBV that lacks BMRF-2 expression by homologous recombination in B95-8 cells. We found that lack of BMRF-2 resulted in about 50% reduction of EBV attachment to oral epithelial cells, but not to B lymphocytes, suggesting that BMRF-2 is critical for EBV infection in oral epithelial cells, but not in B lymphocytes. In polarized oral epithelial cells, infection rate of the recombinant EBV virus was about 4- to 8-fold lower than the wild-type B95-8 virus. Cell adhesion assays using the BMRF-2 RGD peptide and its RGE and AAA mutants showed that the RGD motif is critical for BMRF-2 binding to integrins. These data are consistent with our previous observation that interactions between EBV BMRF-2 and integrins are critical for infection of oral epithelial cells with EBV.
Figures




References
-
- Adam T. Exploitation of host factors for efficient infection by Shigella. Int J Med Microbiol. 2001;291(4):287–98. - PubMed
-
- Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108(3):407–19. - PubMed
-
- Chu JJ, Ng ML. Interaction of West Nile virus with alpha v beta 3 integrin mediates virus entry into cells. J Biol Chem. 2004;279(52):54533–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

