An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: a Gynecologic Oncology Group study
- PMID: 17945336
- PMCID: PMC2699629
- DOI: 10.1016/j.ygyno.2007.09.007
An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: a Gynecologic Oncology Group study
Abstract
Objectives: To investigate the frequency and potential prognostic or predictive value of HER-2 amplification or overexpression in advanced and recurrent endometrial cancers.
Methods: Immunohistochemical staining (IHC; DAKO Herceptest) and fluorescence in situ hybridization (FISH; Vysis Inc. PathVysion DNA Probe Kit) were performed on specimens collected on a randomized Gynecologic Oncology Group (GOG) protocol testing the addition of paclitaxel to doxorubicin/cisplatin.
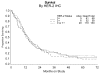
Results: HER-2 overexpression (either 2+ (moderate) or 3+ (strong) immunostaining) and HER-2 gene amplification (a ratio of HER-2 copies to chromosome 17 (CEP17) copies > or = 2) were detected in 44% (104 of 234; 58 were 2+ and 46 were 3+) and 12% (21 of 182) of specimens, respectively. There was a significant increased frequency of overexpression in serous tumors vs. all others (23 of 38, 61% vs. 81 of 196, 41%, respectively, P=0.03). HER-2 amplification also appeared to be more common in serous tumors, but results were not significant (6 of 28, 21% vs. 15 of 141, 11%, P=0.12). There was a significant association between grade and HER-2 amplification among nonserous tumors, with grades 1, 2, and 3 cancers demonstrating 3%, 2%, and 21% amplification, respectively (P=0.003). Neither overexpression nor amplification predicted overall survival (OS) after adjusting for treatment and performance status.
Conclusions: HER-2 amplification was more common in high grade tumors with a trend to being more common in serous tumors. There was no clear evidence for a survival difference or a difference in benefit from the addition of paclitaxel for women with HER-2 amplified or overexpressed tumors; however, power to detect clinically meaningful differences was low.
Figures



References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–30. - PubMed
-
- Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a Gynecology Oncology Group study. J Clin Oncol. 2004;22(19):3902–8. - PubMed
-
- Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2004;22(11):2159–66. - PubMed
-
- Peirâo G, Mayr D, Hillemanns P, Lohrs U, Diebold J. Analysis of HER-2/neu amplification in endometrial carcinoma by Chromogenic in situ hybridization. Correlation with fluorescence in situ hybridization, HER-2/neu, p53 and Ki-67 protein expression and outcome. Mod Pathol. 2004;17(3):227–87. - PubMed
-
- Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15(8):2894–904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

