Plasticity in intact A delta- and C-fibers contributes to cold hypersensitivity in neuropathic rats
- PMID: 17945425
- PMCID: PMC2262053
- DOI: 10.1016/j.neuroscience.2007.09.002
Plasticity in intact A delta- and C-fibers contributes to cold hypersensitivity in neuropathic rats
Abstract
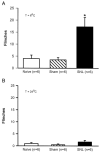
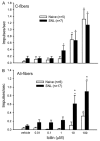
Cold hypersensitivity is a common sensory abnormality accompanying peripheral neuropathies and is difficult to treat. Progress has been made in understanding peripheral mechanisms underlying neuropathic pain but little is known concerning peripheral mechanisms of cold hypersensitivity. The aim of this study was to analyze the contribution of uninjured primary afferents to the cold hypersensitivity that develops in neuropathic rats. Rats with a lumbar 5 (L5) and L6 spinal nerve ligation (SNL, Chung model) but not sham, developed mechanical allodynia, evidenced by decreased paw withdrawal thresholds and increased magnitude of response to von Frey stimulation. Cold hypersensitivity also developed in SNL but not sham rats, evidenced by enhanced nociceptive behaviors induced by placement on a cold plate (6 degrees C) or application of icilin (a transient receptor potential M8 (TRPM8)/transient receptor potential A1 (TRPA1) receptor agonist) to nerve-injured hind paws. Single fiber recordings demonstrated that the mean conduction velocities of intact L4 cutaneous A delta- and C-fibers were not different between naive and SNL rats; however, mechanical thresholds of the A delta- but not the C-fibers were significantly decreased in SNL compared with naive. There was a higher prevalence of C-mechanoheat-cold (CMHC) fibers in SNL compared with naive, but the overall percentage of cold-sensitive C-fibers was not significantly increased compared with naive. This was in contrast to the numerous changes in A delta-fibers: the percentage of L4 cold sensitive A delta-, but not C-fibers, was significantly increased, the percentage of L4 icilin-sensitive A delta-, but not C-fibers, was significantly increased, the icilin-induced activity of L4 A delta-, but not C-fibers, was significantly increased. Icilin-induced activity was blocked by the TRPA1 antagonist Ruthenium Red. The results indicate plasticity in both A delta- and C-uninjured fibers, but A delta fibers appear to provide a major contribution to cold hypersensitivity in neuropathic rats.
Figures







Similar articles
-
Intact Adelta-fibers up-regulate transient receptor potential A1 and contribute to cold hypersensitivity in neuropathic rats.Neuroscience. 2008 Jun 26;154(3):1054-66. doi: 10.1016/j.neuroscience.2008.04.039. Epub 2008 May 2. Neuroscience. 2008. PMID: 18514429 Free PMC article.
-
Comparison of icilin- and cold-evoked responses of spinal neurones, and their modulation of mechanical activity, in a model of neuropathic pain.Brain Res. 2008 Jun 18;1215:87-96. doi: 10.1016/j.brainres.2008.03.072. Epub 2008 Apr 9. Brain Res. 2008. PMID: 18479674
-
Mechanical hyperalgesia after an L5 spinal nerve lesion in the rat is not dependent on input from injured nerve fibers.Pain. 2000 Apr;85(3):493-502. doi: 10.1016/S0304-3959(00)00250-5. Pain. 2000. PMID: 10781924
-
The role of uninjured nerve in spinal nerve ligated rats points to an improved animal model of neuropathic pain.Eur J Pain. 2003;7(5):473-9. doi: 10.1016/S1090-3801(03)00019-3. Eur J Pain. 2003. PMID: 12935800
-
Icilin reduces voltage-gated calcium channel currents in naïve and injured DRG neurons in the rat spinal nerve ligation model.Brain Res. 2014 Apr 4;1557:171-9. doi: 10.1016/j.brainres.2014.02.022. Epub 2014 Feb 18. Brain Res. 2014. PMID: 24560602
Cited by
-
Intact Adelta-fibers up-regulate transient receptor potential A1 and contribute to cold hypersensitivity in neuropathic rats.Neuroscience. 2008 Jun 26;154(3):1054-66. doi: 10.1016/j.neuroscience.2008.04.039. Epub 2008 May 2. Neuroscience. 2008. PMID: 18514429 Free PMC article.
-
Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury.Pain. 2009 Dec 15;147(1-3):265-76. doi: 10.1016/j.pain.2009.09.030. Epub 2009 Oct 22. Pain. 2009. PMID: 19853381 Free PMC article.
-
The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain.PLoS One. 2009 Oct 8;4(10):e7383. doi: 10.1371/journal.pone.0007383. PLoS One. 2009. PMID: 19812688 Free PMC article.
-
Perineural pretreatment of bee venom attenuated the development of allodynia in the spinal nerve ligation injured neuropathic pain model; an experimental study.BMC Complement Altern Med. 2014 Nov 4;14:431. doi: 10.1186/1472-6882-14-431. BMC Complement Altern Med. 2014. PMID: 25366818 Free PMC article.
-
Group II/III metabotropic glutamate receptors exert endogenous activity-dependent modulation of TRPV1 receptors on peripheral nociceptors.J Neurosci. 2011 Sep 7;31(36):12727-37. doi: 10.1523/JNEUROSCI.6558-10.2011. J Neurosci. 2011. PMID: 21900552 Free PMC article.
References
-
- Ali Z, Ringkamp M, Hartke TV, Chien HF, Flavahan NA, Campbell JN, Meyer RA. Uninjured C-fiber nociceptors develop spontaneous activity and α-adrenergic sensitivity following L6 spinal nerve ligation in monkey. J Neurophysiol. 1999;81:455–466. - PubMed
-
- Babes A, Zorzon D, Reid G. A novel type of cold-sensitive neuron in rat dorsal root ganglia with rapid adaptation to cooling stimuli. Eur J Neurosci. 2006;24:691–698. - PubMed
-
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. - PubMed
-
- Baron R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain. 2000;16:S12–S20. - PubMed
-
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

