Gap junction channel structure in the early 21st century: facts and fantasies
- PMID: 17945477
- PMCID: PMC2819411
- DOI: 10.1016/j.ceb.2007.09.001
Gap junction channel structure in the early 21st century: facts and fantasies
Abstract
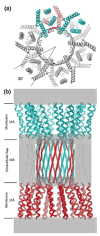
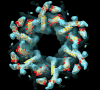
Gap junction channels connect the cytoplasms of adjacent cells through the end-to-end docking of single-membrane structures called connexons, formed by a ring of six connexin monomers. Each monomer contains four transmembrane alpha-helices, for a total of 24 alpha-helices in a connexon. The fundamental structure of the connexon pore is probably similar in unpaired connexons and junctional channels, and for channels formed by different connexin isoforms. Nevertheless, variability in results from structurally focused mutagenesis and electrophysiological studies raise uncertainty about the specific assignments of the transmembrane helices. Mapping of human mutations onto a suggested C(alpha) model predicts that mutations that disrupt helix-helix packing impair channel function. An experimentally determined structure at atomic resolution will be essential to confirm and resolve these concepts.
Figures
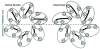
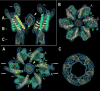
References
-
- Loewenstein WR. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981;61:829–913. - PubMed
-
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. - PubMed
-
- Gerido DA, White TW. Connexin disorders of the ear, skin, and lens. Biochim Biophys Acta. 2004;1662:159–170. - PubMed
-
- Unwin PN, Ennis PD. Two configurations of a channel-forming membrane protein. Nature. 1984;307:609–613. - PubMed
-
- Unger VM, Kumar NM, Gilula NB, Yeager M. Projection structure of a gap junction membrane channel at 7 A resolution. Nat Struct Biol. 1997;4:39–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

