Pituitary gonadotroph estrogen receptor-alpha is necessary for fertility in females
- PMID: 17947360
- PMCID: PMC2194602
- DOI: 10.1210/en.2007-1084
Pituitary gonadotroph estrogen receptor-alpha is necessary for fertility in females
Abstract
Estrogens play a central role in regulating female reproduction throughout the reproductive axis, and the pituitary is one of the major targets of estrogen action. We hypothesized that estrogen receptor alpha (ERalpha) mediates estrogen action in the pituitary gonadotroph. To test this hypothesis, we generated a mouse line with a selective ERalpha deletion in the gonadotropin alpha-subunit (alphaGSU)-expressing pituitary cells (pituitary-specific ERalpha knockout; ERalpha(flox/flox) alphaGSU(cre)). Although the ERalpha(flox/flox) alphaGSU(cre) female mice maintain a basal level of serum LH and FSH and their ovulatory capacity is comparable to that in controls, they do not display regular estrous cycles and are infertile, indicating a potential disorder in regulating LH and/or FSH secretion. The ERalpha(flox/flox) alphaGSU(cre) female mice express equivalent levels of LHbeta and alphaGSU mRNA compared with wild-type mice as determined by microarray analysis. Taken together, these findings indicate that pituitary gonadotroph ERalpha carries out the effects of estrogens with regard to estrous cyclicity and ultimately fertility.
Figures
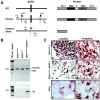


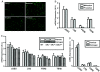
Similar articles
-
The bi-modal effects of estradiol on gonadotropin synthesis and secretion in female mice are dependent on estrogen receptor-alpha.J Endocrinol. 2006 Oct;191(1):309-17. doi: 10.1677/joe.1.06965. J Endocrinol. 2006. PMID: 17065413
-
Estrogen receptor alpha signaling pathways differentially regulate gonadotropin subunit gene expression and serum follicle-stimulating hormone in the female mouse.Endocrinology. 2008 Aug;149(8):4168-76. doi: 10.1210/en.2007-1807. Epub 2008 May 8. Endocrinology. 2008. PMID: 18467444 Free PMC article.
-
Estrogen receptor alpha-induced cholecystokinin type A receptor expression in the female mouse pituitary.J Endocrinol. 2007 Dec;195(3):393-405. doi: 10.1677/JOE-07-0358. J Endocrinol. 2007. PMID: 18000302
-
Discovery of new receptors regulating luteinizing hormone and follicle-stimulating hormone secretion by bovine gonadotrophs to explore a new paradigm for mechanisms regulating reproduction.J Reprod Dev. 2020 Aug 20;66(4):291-297. doi: 10.1262/jrd.2020-012. Epub 2020 Apr 6. J Reprod Dev. 2020. PMID: 32249236 Free PMC article. Review.
-
Possible role of PACAP and its PAC1 receptor in the differential regulation of pituitary LHbeta- and FSHbeta-subunit gene expression by pulsatile GnRH stimulation.Biol Reprod. 2013 Feb 14;88(2):35. doi: 10.1095/biolreprod.112.105601. Print 2013 Feb. Biol Reprod. 2013. PMID: 23197164 Review.
Cited by
-
Estrogen Biosynthesis and Signal Transduction in Ovarian Disease.Front Endocrinol (Lausanne). 2022 Mar 1;13:827032. doi: 10.3389/fendo.2022.827032. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35299973 Free PMC article. Review.
-
Inverse association between estrogen receptor-α DNA methylation and breast composition in adolescent Chilean girls.Clin Epigenetics. 2018 Oct 4;10(1):122. doi: 10.1186/s13148-018-0553-5. Clin Epigenetics. 2018. PMID: 30286806 Free PMC article.
-
Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance.Diabetes. 2013 Feb;62(2):424-34. doi: 10.2337/db11-1718. Epub 2012 Sep 10. Diabetes. 2013. PMID: 22966069 Free PMC article.
-
Hematopoetic prostaglandin D synthase: an ESR1-dependent oviductal epithelial cell synthase.Endocrinology. 2012 Apr;153(4):1925-35. doi: 10.1210/en.2011-1900. Epub 2012 Feb 28. Endocrinology. 2012. PMID: 22374975 Free PMC article.
-
Cross-Species Transcriptomics Analysis Highlights Conserved Molecular Responses to Per- and Polyfluoroalkyl Substances.Toxics. 2023 Jun 29;11(7):567. doi: 10.3390/toxics11070567. Toxics. 2023. PMID: 37505532 Free PMC article.
References
-
- Clarke IJ 2002 Multifarious effects of estrogen on the pituitary gonadotrope with special emphasis on studies in the ovine species. Arch Physiol Biochem 110:62–73 - PubMed
-
- Levine JE 1997 New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod 56:293–302 - PubMed
-
- Knobil E, Neill JD 1988 The physiology of reproduction. New York: Raven Press
-
- Nett TM, Turzillo AM, Baratta M, Rispoli LA 2002 Pituitary effects of steroid hormones on secretion of follicle-stimulating hormone and luteinizing hormone. Domest Anim Endocrinol 23:33–42 - PubMed
-
- Yin P, Kawashima K, Arita J 2002 Direct actions of estradiol on the anterior pituitary gland are required for hypothalamus-dependent lactotrope proliferation and secretory surges of luteinizing hormone but not of prolactin in female rats. Neuroendocrinology 75:392–401 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

