Histone acetylation at the Ifng promoter in tolerized CD4 cells is associated with increased IFN-gamma expression during subsequent immunization to the same antigen
- PMID: 17947638
- PMCID: PMC2855051
- DOI: 10.4049/jimmunol.179.9.5669
Histone acetylation at the Ifng promoter in tolerized CD4 cells is associated with increased IFN-gamma expression during subsequent immunization to the same antigen
Abstract
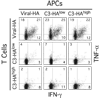
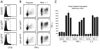
When naive CD4(+) Th cells encounter cognate pathogen-derived Ags they expand and develop the capacity to express the appropriate effector cytokines for neutralizing the pathogen. Central to this differentiation process are epigenetic modifications within the effector cytokine genes that allow accessibility to the transcriptional machinery. In contrast, when mature self-reactive CD4 cells encounter their cognate epitopes in the periphery they generally undergo a process of tolerization in which they become hyporesponsive/anergic to antigenic stimulation. In the current study, we used a TCR transgenic adoptive transfer system to demonstrate that in a dose-dependent manner parenchymal self-Ag programs cognate naive CD4 cells to acetylate histones bound to the promoter region of the Ifng gene (which encodes the signature Th1 effector cytokine) during peripheral tolerization. Although the Ifng gene gains transcriptional competence, these tolerized CD4 cells fail to express substantial amounts of IFN-gamma in response to antigenic stimulation apparently because a blockage in TCR-mediated signaling also develops. Nevertheless, responsiveness to antigenic stimulation is partially restored when self-Ag-tolerized CD4 cells are retransferred into mice infected with a virus expressing the same Ag. Additionally, there is preferential boosting in the ability of these CD4 cells to express IFN-gamma relative to other cytokines with expression that also becomes impaired. Taken together, these results suggest that epigenetic modification of the Ifng locus during peripheral CD4 cell tolerization might allow for preferential expression of IFN-gamma during recovery from tolerance.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures




Similar articles
-
Self-antigen prevents CD8 T cell effector differentiation by CD134 and CD137 dual costimulation.J Immunol. 2008 Dec 1;181(11):7728-37. doi: 10.4049/jimmunol.181.11.7728. J Immunol. 2008. PMID: 19017962 Free PMC article.
-
T-bet down-modulation in tolerized Th1 effector CD4 cells confers a TCR-distal signaling defect that selectively impairs IFN-gamma expression.J Immunol. 2006 Jan 15;176(2):1036-45. doi: 10.4049/jimmunol.176.2.1036. J Immunol. 2006. PMID: 16393991 Free PMC article.
-
Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells.J Immunol. 2006 Jul 15;177(2):1062-9. doi: 10.4049/jimmunol.177.2.1062. J Immunol. 2006. PMID: 16818762
-
Epigenetic control of interferon-gamma expression in CD8 T cells.J Immunol Res. 2015;2015:849573. doi: 10.1155/2015/849573. Epub 2015 Apr 20. J Immunol Res. 2015. PMID: 25973438 Free PMC article. Review.
-
Regulation of interferon-gamma during innate and adaptive immune responses.Adv Immunol. 2007;96:41-101. doi: 10.1016/S0065-2776(07)96002-2. Adv Immunol. 2007. PMID: 17981204 Review.
Cited by
-
Self-antigen prevents CD8 T cell effector differentiation by CD134 and CD137 dual costimulation.J Immunol. 2008 Dec 1;181(11):7728-37. doi: 10.4049/jimmunol.181.11.7728. J Immunol. 2008. PMID: 19017962 Free PMC article.
-
CD8+ dendritic cell-mediated tolerance of autoreactive CD4+ T cells is deficient in NOD mice and can be corrected by blocking CD40L.J Leukoc Biol. 2014 Feb;95(2):325-36. doi: 10.1189/jlb.0113013. Epub 2013 Sep 30. J Leukoc Biol. 2014. PMID: 24082013 Free PMC article.
-
A Two-Step Process of Effector Programming Governs CD4+ T Cell Fate Determination Induced by Antigenic Activation in the Steady State.Cell Rep. 2020 Nov 24;33(8):108424. doi: 10.1016/j.celrep.2020.108424. Cell Rep. 2020. PMID: 33238127 Free PMC article.
-
Applications of Antibody-Based Antigen Delivery Targeted to Dendritic Cells In Vivo.Antibodies (Basel). 2022 Jan 25;11(1):8. doi: 10.3390/antib11010008. Antibodies (Basel). 2022. PMID: 35225867 Free PMC article. Review.
-
The E3 ubiquitin ligase Cbl-b regulates expansion but not functional activity of self-reactive CD4 T cells.J Immunol. 2009 Oct 15;183(8):4975-83. doi: 10.4049/jimmunol.0901243. J Immunol. 2009. PMID: 19801520 Free PMC article.
References
-
- Murphy KM, Reiner SL. Decision making in the immune system: The lineage decisions of helper T cells. Nat. Rev. Immunol. 2002;2:933–944. - PubMed
-
- Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat. Rev. Immunol. 2004;4:900–911. - PubMed
-
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. - PubMed
-
- Pulendran B. Variegation of the immune response with dendritic cells and pathogen recognition receptors. J. Immunol. 2005;174:2457–2465. - PubMed
-
- Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

