Neuropathogical features of a rat model for perinatal hypoxic-ischemic encephalopathy with associated epilepsy
- PMID: 17948865
- PMCID: PMC4607042
- DOI: 10.1002/cne.21533
Neuropathogical features of a rat model for perinatal hypoxic-ischemic encephalopathy with associated epilepsy
Abstract
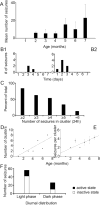
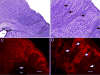
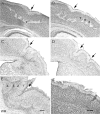
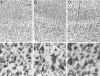
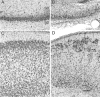
Hypoxic-ischemic (HI) encephalopathy is an important neurological problem of the perinatal period. Little is known of the long-term progression of HI insults or the maladaptive changes that lead to epilepsy. Using rats with unilateral carotid occlusion followed by hypoxia at postnatal day 7, this study provides an initial analysis of the epilepsy caused by a perinatal HI insult with chronic and continuous behavioral monitoring. The histopathology was investigated at postnatal day 30 and later at > or =6 months of age using cresyl violet, Timm, and rapid Golgi staining and immunocytochemistry. The resultant epilepsy showed an increase in seizure frequency over time, with a preponderance for seizure clusters and behavioral features of an ipsilateral cerebral syndrome. In addition to parasagittal infarcts and porencephalic cysts in severe lesions, columnar neuronal death was found with cytomegaly in isolated groups of dysmorphic cortical neurons. Cortical dysgenesis was seen in the form of deep laminar cell loss, microgyri, white matter hypercellularity, and blurring of the white and gray matter junction. Mossy fiber sprouting was not only detected in the atrophied ipsilateral dorsal hippocampus of HI rats with chronic epilepsy, but was also found in comparable grades in spared ipsi- and contralateral ventral hippocampi. The cortical lesions in this animal model show histological similarities with those found in humans after perinatal HI. The occurrence of cortical abnormalities that are associated with epilepsy in humans correlates with the consequent detection of spontaneous recurrent seizures.
Figures






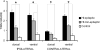
Similar articles
-
Continuous electroencephalographic monitoring with radio-telemetry in a rat model of perinatal hypoxia-ischemia reveals progressive post-stroke epilepsy.J Neurosci. 2010 Jan 6;30(1):404-15. doi: 10.1523/JNEUROSCI.4093-09.2010. J Neurosci. 2010. PMID: 20053921 Free PMC article.
-
A chronic histopathological and electrophysiological analysis of a rodent hypoxic-ischemic brain injury model and its use as a model of epilepsy.Neuroscience. 2007 Nov 23;149(4):943-61. doi: 10.1016/j.neuroscience.2007.07.067. Epub 2007 Sep 12. Neuroscience. 2007. PMID: 17935893 Free PMC article.
-
Epilepsy and synaptic reorganization in a perinatal rat model of hypoxia-ischemia.Epilepsia. 2004 Oct;45(10):1210-8. doi: 10.1111/j.0013-9580.2004.60403.x. Epilepsia. 2004. PMID: 15461675
-
The role of glutamate receptor maturation in perinatal seizures and brain injury.Int J Dev Neurosci. 2002 Jun-Aug;20(3-5):339-47. doi: 10.1016/s0736-5748(02)00012-6. Int J Dev Neurosci. 2002. PMID: 12175872 Review.
-
Cortical malformations and epilepsy: new insights from animal models.Epilepsia. 1999 Jul;40(7):811-21. doi: 10.1111/j.1528-1157.1999.tb00786.x. Epilepsia. 1999. PMID: 10403203 Review.
Cited by
-
Brain Distribution and Metabolism of Flupirtine, a Nonopioid Analgesic Drug with Antiseizure Effects, in Neonatal Rats.Pharmaceutics. 2018 Dec 16;10(4):281. doi: 10.3390/pharmaceutics10040281. Pharmaceutics. 2018. PMID: 30558371 Free PMC article.
-
Sleep dysfunction following neonatal ischemic seizures are differential by neonatal age of insult as determined by qEEG in a mouse model.Neurobiol Dis. 2018 Aug;116:1-12. doi: 10.1016/j.nbd.2018.04.012. Epub 2018 Apr 21. Neurobiol Dis. 2018. PMID: 29684437 Free PMC article.
-
Background suppression of electrical activity is a potential biomarker of subsequent brain injury in a rat model of neonatal hypoxia-ischemia.J Neurophysiol. 2022 Jul 1;128(1):118-130. doi: 10.1152/jn.00024.2022. Epub 2022 Jun 8. J Neurophysiol. 2022. PMID: 35675445 Free PMC article.
-
A model of posttraumatic epilepsy after penetrating brain injuries: effect of lesion size and metal fragments.Epilepsia. 2014 Dec;55(12):1969-77. doi: 10.1111/epi.12854. Epub 2014 Dec 3. Epilepsia. 2014. PMID: 25470332 Free PMC article.
-
Electroacupuncture at Baihui (DU20) acupoint up-regulates mRNA expression of NeuroD molecules in the brains of newborn rats suffering in utero fetal distress.Neural Regen Res. 2016 Apr;11(4):604-9. doi: 10.4103/1673-5374.180745. Neural Regen Res. 2016. PMID: 27212921 Free PMC article.
References
-
- Alonso-Nanclares L, Garbelli R, Sola RG, Pastor J, Tassi L, Spreafico R, DeFelipe J. Microanatomy of the dysplastic neocortex from epileptic patients. Brain. 2005;128:158–173. - PubMed
-
- Andres M, Andre VM, Nguyen S, Salamon N, Cepeda C, Levine MS, Leite JP, Neder L, Vinters HV, Mathern GW. Human cortical dysplasia and epilepsy: an ontogenetic hypothesis based on volumetric MRI and NeuN neuronal density and size measurements. Cereb Cortex. 2005;15:194–210. - PubMed
-
- Andrews RJ. Transhemispheric diaschisis. A review and comment. Stroke. 1991;22:943–949. - PubMed
-
- Ansari MQ, Chincanchan CA, Armstrong DL. Brain calcification in hypoxic-ischemic lesions: an autopsy review. Pediatr Neurol. 1990;6:94–101. - PubMed
-
- Armstrong DD, Mizrahi EM. Pathologic basis of the symptomatic epilepsies in childhood. J Child Neurol. 1998;13:361–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

