Rational prescription of drugs within similar therapeutic or structural class for gastrointestinal disease treatment: drug metabolism and its related interactions
- PMID: 17948937
- PMCID: PMC4172742
- DOI: 10.3748/wjg.v13.i42.5618
Rational prescription of drugs within similar therapeutic or structural class for gastrointestinal disease treatment: drug metabolism and its related interactions
Abstract
Aim: To review and summarize drug metabolism and its related interactions in prescribing drugs within the similar therapeutic or structural class for gastrointestinal disease treatment so as to promote rational use of medicines in clinical practice.
Methods: Relevant literature was identified by performing MEDLINE/Pubmed searches covering the period from 1988 to 2006.
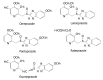
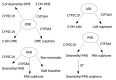
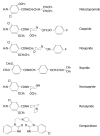
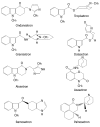
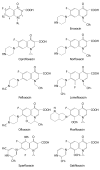
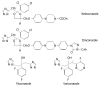
Results: Seven classes of drugs were chosen, including gastric proton pump inhibitors, histamine H(2)-receptor antagonists, benzamide-type gastroprokinetic agents, selective 5-HT(3) receptor antagonists, fluoroquinolones, macrolide antibiotics and azole antifungals. They showed significant differences in metabolic profile (i.e., the fraction of drug metabolized by cytochrome P450 (CYP), CYP reaction phenotype, impact of CYP genotype on interindividual pharmacokinetics variability and CYP-mediated drug-drug interaction potential). Many events of severe adverse drug reactions and treatment failures were closely related to the ignorance of the above issues.
Conclusion: Clinicians should acquaint themselves with what kind of drug has less interpatient variability in clearance and whether to perform CYP genotyping prior to initiation of therapy. The relevant CYP knowledge helps clinicians to enhance the management of patients with gastrointestinal disease who may require treatment with polytherapeutic regimens.
Figures


Similar articles
-
MDR- and CYP3A4-mediated drug-drug interactions.J Neuroimmune Pharmacol. 2006 Sep;1(3):323-39. doi: 10.1007/s11481-006-9034-2. Epub 2006 Aug 2. J Neuroimmune Pharmacol. 2006. PMID: 18040809 Review.
-
New pharmacologic therapies in gastrointestinal disease.Gastroenterol Clin North Am. 2010 Sep;39(3):709-20. doi: 10.1016/j.gtc.2010.08.020. Gastroenterol Clin North Am. 2010. PMID: 20951926 Review.
-
A review of medical therapy for proton pump inhibitor nonresponsive gastroesophageal reflux disease.Dis Esophagus. 2017 Sep 1;30(9):1-15. doi: 10.1093/dote/dox055. Dis Esophagus. 2017. PMID: 28859358 Free PMC article. Review.
-
Gastrointestinal medications.Psychosomatics. 2007 Jan-Feb;48(1):79-85. doi: 10.1176/appi.psy.48.1.79. Psychosomatics. 2007. PMID: 17209156 Review.
-
Review article: drug interactions with agents used to treat acid-related diseases.Aliment Pharmacol Ther. 1999 Aug;13 Suppl 3:18-26. doi: 10.1046/j.1365-2036.1999.00021.x. Aliment Pharmacol Ther. 1999. PMID: 10491725 Review.
Cited by
-
Interspecies Variation in NCMN-O-Demethylation in Liver Microsomes from Various Species.Molecules. 2019 Jul 30;24(15):2765. doi: 10.3390/molecules24152765. Molecules. 2019. PMID: 31366067 Free PMC article.
-
Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine.Expert Opin Drug Metab Toxicol. 2018 Apr;14(4):447-460. doi: 10.1080/17425255.2018.1461835. Epub 2018 Apr 12. Expert Opin Drug Metab Toxicol. 2018. PMID: 29620484 Free PMC article. Review.
-
The association between gastric acid inhibitors and delirium in geriatric inpatients: implications for clinical practice and research.Clin Interv Aging. 2016 Jun 16;11:797-9. doi: 10.2147/CIA.S111202. eCollection 2016. Clin Interv Aging. 2016. PMID: 27366056 Free PMC article. No abstract available.
-
Drug utilization of clarithromycin for gastrointestinal disease treatment.World J Gastroenterol. 2008 Oct 21;14(39):6065-71. doi: 10.3748/wjg.14.6065. World J Gastroenterol. 2008. PMID: 18932287 Free PMC article.
-
The epidemiology and phenomenology of non-antipsychotic-induced dystonia: a hybrid systematic-narrative review.Eur Psychiatry. 2025 Feb 10;68(1):e36. doi: 10.1192/j.eurpsy.2025.18. Eur Psychiatry. 2025. PMID: 39925222 Free PMC article.
References
-
- Ansede JH, Thakker DR. High-throughput screening for stability and inhibitory activity of compounds toward cytochrome P450-mediated metabolism. J Pharm Sci. 2004;93:239–255. - PubMed
-
- Lewis DF, Modi S, Dickins M. Quantitative structure-activity relationships (QSARs) within substrates of human cytochromes P450 involved in drug metabolism. Drug Metabol Drug Interact. 2001;18:221–242. - PubMed
-
- Lewis DF, Modi S, Dickins M. Structure-activity relationship for human cytochrome P450 substrates and inhibitors. Drug Metab Rev. 2002;34:69–82. - PubMed
-
- FDA Guidance for industry drug metabolism/drug interaction studies in the drug development process: studies in vitro. April 1997. Available from: http: //www.fda.gov/cder/guidance/clin3.pdf.
-
- FDA Guidance for Industry In Vivo Drug Metabolism/Drug Interaction Studies -Study Design, Data Analysis, and Recommendations for Dosing and Labeling. November 1999. Available from: http: //www.fda.gov/cber/gdlns/metabol.htm.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

