Multiple G-protein-coupling specificity of beta-adrenoceptor in macrophages
- PMID: 17949419
- PMCID: PMC2266041
- DOI: 10.1111/j.1365-2567.2007.02658.x
Multiple G-protein-coupling specificity of beta-adrenoceptor in macrophages
Abstract
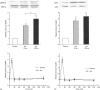
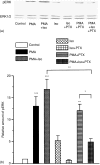
Adrenergic signalling of the immune system is one of the important modulator pathways of the inflammatory immune response realized via G protein-mediated pathways. The resulted signal depends on the type of the receptor-coupled G-protein (GPCR) that, according to the classical paradigm in the case of beta-adrenergic receptor (beta-AR), is Gs-type. Recently, alternate and/or multiple G protein coupling specificity of GPCRs have been demonstrated including a switch from Gs to Gi binding. The possibility of a Gs/Gi switch and its role in the immune response of macrophages has not been investigated yet. In this study, we demonstrate that beta-adrenergic stimulation itself is able to induce a transient mitogen-activated protein kinase phosphorylation in murine peritoneal macrophages in a pertussis toxin-sensitive manner, suggesting that the Gs/Gi switch also occurs in the immune system. Although this process is very rapid, it can influence different signalling pathways and can reprogramme effector functions suggesting that sympathetic modulation of the defence mechanism of the innate immune system has an additional, Gs/Gi switch-dependent component.
Figures
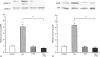

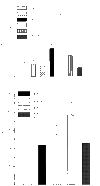
Similar articles
-
Duality of G protein-coupled mechanisms for beta-adrenergic activation of NKCC activity in skeletal muscle.Am J Physiol Cell Physiol. 2002 Oct;283(4):C1025-32. doi: 10.1152/ajpcell.00096.2002. Am J Physiol Cell Physiol. 2002. PMID: 12225966
-
Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A.Nature. 1997 Nov 6;390(6655):88-91. doi: 10.1038/36362. Nature. 1997. PMID: 9363896
-
The role of Gi proteins in reduced vasorelaxation response to beta-adrenoceptor agonists in rat aorta during maturation.Eur J Pharmacol. 2007 Jun 14;564(1-3):167-73. doi: 10.1016/j.ejphar.2007.02.054. Epub 2007 Mar 7. Eur J Pharmacol. 2007. PMID: 17395174
-
Multiple pathways of ERK activation by G protein-coupled receptors.Novartis Found Symp. 2001;239:68-79; discussion 80-4, 150-9. doi: 10.1002/0470846674.ch7. Novartis Found Symp. 2001. PMID: 11529317 Review.
-
Adrenergic regulation of myocardial apoptosis.Cardiovasc Res. 2000 Feb;45(3):713-9. doi: 10.1016/s0008-6363(99)00370-3. Cardiovasc Res. 2000. PMID: 10728393 Review.
Cited by
-
Activation of p38 mitogen-activated protein kinase by norepinephrine in T-lineage cells.Immunology. 2011 Feb;132(2):197-208. doi: 10.1111/j.1365-2567.2010.03354.x. Epub 2010 Oct 13. Immunology. 2011. PMID: 21039464 Free PMC article.
-
Proinflammatory switch from Gαs to Gαi signaling by Glucagon-like peptide-1 receptor in murine splenic monocyte following burn injury.Inflamm Res. 2018 Feb;67(2):157-168. doi: 10.1007/s00011-017-1104-9. Epub 2017 Oct 11. Inflamm Res. 2018. PMID: 29022064
-
Recruitment of β-arrestin 1 and 2 to the β2-adrenoceptor: analysis of 65 ligands.J Pharmacol Exp Ther. 2015 Nov;355(2):183-90. doi: 10.1124/jpet.115.227959. Epub 2015 Aug 25. J Pharmacol Exp Ther. 2015. PMID: 26306764 Free PMC article.
-
Adrenoceptors in the Eye - Physiological and Pathophysiological Relevance.Handb Exp Pharmacol. 2024;285:453-505. doi: 10.1007/164_2023_702. Handb Exp Pharmacol. 2024. PMID: 38082203 Review.
-
Substance P and Alpha-Calcitonin Gene-Related Peptide Differentially Affect Human Osteoarthritic and Healthy Chondrocytes.Front Immunol. 2021 Aug 27;12:722884. doi: 10.3389/fimmu.2021.722884. eCollection 2021. Front Immunol. 2021. PMID: 34512650 Free PMC article.
References
-
- Besedovsky H, Sorkin E, Keller M, Muller J. Changes in blood hormone levels during the immune response. Proc Soc Exp Biol Med Soc Exp Biol Med (New York, N Y) 1975;150:466. - PubMed
-
- Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel SL. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol. 1990;145:1430. - PubMed
-
- Felten SY, Olschowka J. Noradrenergic sympathetic innervation of the spleen. II. Tyrosine hydroxylase (TH) -positive nerve terminals form synaptic like contacts on lymphocytes in the splenic white pulp. J Neurosci Res. 1987;18:37. - PubMed
-
- Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, Olschowki JA, Livnat S. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987;100:225. - PubMed
-
- Vizi ES. Receptor-mediated local fine-tuning by noradrenergic innervation of neuroendocrine and immune systems. Ann N Y Acad Sci. 1998;851:388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

