Screening of potential a disintegrin and metalloproteinase with thrombospondin motifs-4 inhibitors using a collagen model fluorescence resonance energy transfer substrate
- PMID: 17949675
- PMCID: PMC2245870
- DOI: 10.1016/j.ab.2007.09.014
Screening of potential a disintegrin and metalloproteinase with thrombospondin motifs-4 inhibitors using a collagen model fluorescence resonance energy transfer substrate
Abstract
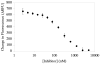
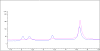
The major components of the cartilage extracellular matrix are type II collagen and aggrecan. Type II collagen provides cartilage with its tensile strength, whereas the water-binding capacity of aggrecan provides compressibility and elasticity. Aggrecan breakdown leads to an increase in proteolytic susceptibility of articular collagen; hence, aggrecan may also have a protective effect on type II collagen. Given their role in aggrecan degradation and differing substrate specificity profiles, the pursuit of inhibitors for both aggrecanase 1 (a disintegrin and metalloproteinase with thrombospondin motifs-4 [ADAMTS-4]) and aggrecanase 2 (ADAMTS-5) is desirable. We previously described collagen model fluorescence resonance energy transfer (FRET) substrates for aggrecan-degrading members of the ADAMTS family. These FRET substrate assays are also fully compatible with multiwell formats. In the current study, a collagen model FRET substrate was examined for inhibitor screening of ADAMTS-4. ADAMTS-4 was screened against a small compound library (n=960) with known pharmacological activity. Five compounds that inhibited ADAMTS-4>60% at a concentration of 1muM were identified. A secondary screen using reversed-phase high-performance liquid chromatography (RP-HPLC) was developed and performed for verification of the five potential inhibitors. Ultimately, piceatannol was confirmed as a novel inhibitor of ADAMTS-4, with an IC(50) value of 1muM. Because the collagen model FRET substrates have distinct conformational features that may interact with protease secondary substrate sites (exosites), nonactive site-binding inhibitors can be identified via this approach. Selective inhibitors for ADAMTS-4 would allow a more definitive evaluation of this protease in osteoarthritis and also represent a potential next generation in metalloproteinase therapeutics.
Figures






Similar articles
-
Aggrecanase and aggrecan degradation in osteoarthritis: a review.J Int Med Res. 2008 Nov-Dec;36(6):1149-60. doi: 10.1177/147323000803600601. J Int Med Res. 2008. PMID: 19094423 Review.
-
TIMP-3 inhibition of ADAMTS-4 (Aggrecanase-1) is modulated by interactions between aggrecan and the C-terminal domain of ADAMTS-4.J Biol Chem. 2007 Jul 20;282(29):20991-8. doi: 10.1074/jbc.M610721200. Epub 2007 Apr 30. J Biol Chem. 2007. PMID: 17470431
-
Identification of potent and selective hydantoin inhibitors of aggrecanase-1 and aggrecanase-2 that are efficacious in both chemical and surgical models of osteoarthritis.J Med Chem. 2014 Dec 26;57(24):10476-85. doi: 10.1021/jm501522n. Epub 2014 Dec 5. J Med Chem. 2014. PMID: 25415648
-
The effect of protease inhibitors on the induction of osteoarthritis-related biomarkers in bovine full-depth cartilage explants.PLoS One. 2015 Apr 24;10(4):e0122700. doi: 10.1371/journal.pone.0122700. eCollection 2015. PLoS One. 2015. PMID: 25909781 Free PMC article.
-
Drug insight: aggrecanases as therapeutic targets for osteoarthritis.Nat Clin Pract Rheumatol. 2008 Aug;4(8):420-7. doi: 10.1038/ncprheum0841. Epub 2008 Jun 24. Nat Clin Pract Rheumatol. 2008. PMID: 18577998 Review.
Cited by
-
Using fluorogenic peptide substrates to assay matrix metalloproteinases.Methods Mol Biol. 2001;151:495-518. doi: 10.1385/1-59259-046-2:495. Methods Mol Biol. 2001. PMID: 11217324 Free PMC article.
-
Analysis of flavonoid-based pharmacophores that inhibit aggrecanases (ADAMTS-4 and ADAMTS-5) and matrix metalloproteinases through the use of topologically constrained peptide substrates.Chem Biol Drug Des. 2009 Nov;74(5):473-82. doi: 10.1111/j.1747-0285.2009.00885.x. Epub 2009 Sep 28. Chem Biol Drug Des. 2009. PMID: 19793184 Free PMC article.
-
SKI306X inhibition of glycosaminoglycan degradation in human cartilage involves down-regulation of cytokine-induced catabolic genes.Korean J Intern Med. 2014 Sep;29(5):647-55. doi: 10.3904/kjim.2014.29.5.647. Epub 2014 Aug 28. Korean J Intern Med. 2014. PMID: 25228841 Free PMC article.
-
High throughput screening of potentially selective MMP-13 exosite inhibitors utilizing a triple-helical FRET substrate.Bioorg Med Chem. 2009 Feb 1;17(3):990-1005. doi: 10.1016/j.bmc.2008.03.004. Epub 2008 Mar 6. Bioorg Med Chem. 2009. PMID: 18358729 Free PMC article.
-
A high-throughput FRET-based assay for determination of Atg4 activity.Autophagy. 2012 Mar;8(3):401-12. doi: 10.4161/auto.18777. Epub 2012 Feb 3. Autophagy. 2012. PMID: 22302004 Free PMC article.
References
-
- Felson DT. The epidemiology of OA: Results from the Framingham OA study. Seminars Arthritis Rheum. 1990;20:42–50. - PubMed
-
- Knudson CB, Knudson W. Cartilage proteoglycans. Seminars Cell Dev Biol. 2001;12:69–78. - PubMed
-
- Pratta M, Yao W, Decicco C, Tortorella MD, Liu RW, Copeland RA, Magolda R, Newton RC, Trzaskos JM, Arner EC. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278:45539–45545. - PubMed
-
- Wang J, Verdonk P, Elewaut D, Yveys EM, Verbruggen G. Homeostasis of the ECM of normal and OA human articular cartilage chondrocytes in vitro. Osteoarthritis Cartilage. 2003;11:801–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

