Histone modifications influence the action of Snf2 family remodelling enzymes by different mechanisms
- PMID: 17949749
- PMCID: PMC2279226
- DOI: 10.1016/j.jmb.2007.09.059
Histone modifications influence the action of Snf2 family remodelling enzymes by different mechanisms
Abstract
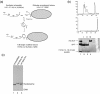
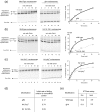
Alteration of chromatin structure by chromatin modifying and remodelling activities is a key stage in the regulation of many nuclear processes. These activities are frequently interlinked, and many chromatin remodelling enzymes contain motifs that recognise modified histones. Here we adopt a peptide ligation strategy to generate specifically modified chromatin templates and used these to study the interaction of the Chd1, Isw2 and RSC remodelling complexes with differentially acetylated nucleosomes. Specific patterns of histone acetylation are found to alter the rate of chromatin remodelling in different ways. For example, histone H3 lysine 14 acetylation acts to increase recruitment of the RSC complex to nucleosomes. However, histone H4 tetra-acetylation alters the spectrum of remodelled products generated by increasing octamer transfer in trans. In contrast, histone H4 tetra-acetylation was also found to reduce the activity of the Chd1 and Isw2 remodelling enzymes by reducing catalytic turnover without affecting recruitment. These observations illustrate a range of different means by which modifications to histones can influence the action of remodelling enzymes.
Figures





References
-
- Durr H., Korner C., Muller M., Hickmann V., Hopfner K.P. X-ray structures of the Sulfolobus solfataricus SWI2/SNF2 ATPase core and its complex with DNA. Cell. 2005;121:363–373. - PubMed
-
- Thoma N.H., Czyzewski B.K., Alexeev A.A., Mazin A.V., Kowalczykowski S.C., Pavletich N.P. Structure of the SWI2/SNF2 chromatin-remodeling domain of eukaryotic Rad54. Nature Struct. Mol. Biol. 2005;12:350–356. - PubMed
-
- Saha A., Wittmeyer J., Cairns B.R. Chromatin remodelling: the industrial revolution of DNA around histones. Nature Rev. Mol. Cell Biol. 2006;7:437–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

