KIT regulates tyrosine phosphorylation and nuclear localization of beta-catenin in mast cell leukemia
- PMID: 17949810
- PMCID: PMC2682210
- DOI: 10.1016/j.leukres.2007.08.023
KIT regulates tyrosine phosphorylation and nuclear localization of beta-catenin in mast cell leukemia
Abstract
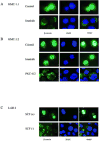
Gain-of-function mutations in the proto-oncogene c-kit that induce constitutive kinase activity of its product, KIT protein, are characteristic of human mast cell disease and are believed to play a central role in mast cell leukemia oncogenesis, proliferation and survival. Nuclear overexpression of the Wnt effector beta-catenin and deregulated beta-catenin nuclear signaling can promote malignant transformation in solid tumors and hematologic malignancies. However, a role for beta-catenin in mast cell leukemia has not been described. Nuclear accumulation of beta-catenin is upregulated by its tyrosine phosphorylation, a process that can be exacerbated by deregulated expression of oncogenic tyrosine kinases. Here, we investigated the relationship between activated KIT and beta-catenin signaling in mast cell leukemia. Beta-catenin was tyrosine-phosphorylated in cells with KIT activated by either gain-of-function mutation or incubation with the KIT ligand stem cell factor. Beta-catenin tyrosine phosphorylation depended on KIT activity but not on PI3K-AKT activation. Tyrosine phosphorylation of beta-catenin was associated with its nuclear localization and enhanced transcription of target genes c-myc and cyclin D1. Endogenous KIT and beta-catenin were found to associate in mast cell leukemia cells, and in vitro kinase assay demonstrated that active KIT phosphorylates tyrosine residues of beta-catenin directly. Aberrant beta-catenin-driven transcription caused by deregulated KIT may represent a significant new target for treatment of mast cell leukemia.
Figures




References
-
- Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990;63:235–43. - PubMed
-
- Martin FH, Suggs SV, Langley KE, Lu HS, Ting J, Okino KH, et al. Primary structure and functional expression of rat and human stem cell factor DNAs. Cell. 1990;63:203–11. - PubMed
-
- Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–20. - PubMed
-
- Blechman JM, Lev S, Givol D, Yarden Y. Structure-function analyses of the kit receptor for the steel factor. Stem Cells. 1993;11 (Suppl 2):12–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

