Stimulation of transactivation of the largemouth bass estrogen receptors alpha, beta-a, and beta-b by methoxychlor and its mono- and bis-demethylated metabolites in HepG2 cells
- PMID: 17949972
- PMCID: PMC2268757
- DOI: 10.1016/j.jsbmb.2007.06.004
Stimulation of transactivation of the largemouth bass estrogen receptors alpha, beta-a, and beta-b by methoxychlor and its mono- and bis-demethylated metabolites in HepG2 cells
Abstract
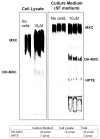
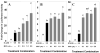
The purpose of this study was to determine the mechanisms by which the pesticide, methoxychlor (MXC), acts as an environmental endocrine disruptor through interaction with the three largemouth bass (Micropterus salmoides) estrogen receptors (ERs) alpha, betaa, and betab. MXC is a less-environmentally persistent analog of DDT that behaves as a weak estrogen. Using transient transfection assays in HepG2 cells, we have previously shown that each receptor is responsive to the endogenous ligand 17beta-estradiol (E(2)) in a dose-dependent manner. The parent compound, MXC, showed dose-dependent stimulation of transcriptional activation through all three ERs. In addition to the parent molecule, each of the metabolites was also estrogenic with all three ERs. The order of potency for ERalpha and ERbetab was HPTE>OH-MXC>MXC, while the opposite order was seen for ERbetaa. HepG2 cells did not substantially metabolize MXC to the active metabolites, thus the activity of MXC was not due to metabolism. When examining the effects of increasing concentrations of MXC at a fixed concentration of E(2), all three ERs show increased activity compared to that with E(2) alone, showing that the effects of MXC and E(2) are additive. However, when this experiment was repeated with increasing concentrations of HPTE at a fixed concentration of E(2), the activity of ERalpha was decreased, that of ERbetab was increased, while that of ERbetaa was unaffected compared to E(2) alone. These experiments suggest that HPTE functions as an E(2) antagonist with ERalpha, an E(2) agonist with ERbetab and does not perturb E(2) stimulation of ERbetaa. While it is clear the ERbeta subtypes are the products of different genes (due to a gene duplication in teleosts) the differences in their responses to MXC and its metabolites indicate that their functions diverge, both in their in vivo molecular response to E(2), as well as in their interaction with endocrine disrupting compounds found in the wild.
Figures



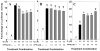
References
-
- Ing NH, O’Malley BW. The steroid hormone receptor superfamily- molecular mechanisms of action. In: Weintraub B, editor. Molecular Endocrinology: Basic Concepts and Clinical Correlations. Raven Press; New York: 1995. pp. 195–215.
-
- Augustijn-Beckers PWM, Hornsby AG, Wauchope RD. SCS/ARS/CES pesticide properties database for environmental decision-making II: Additional compounds. Rev Environ Cont Tox. 1994;137:1–82. - PubMed
-
- EPA. Environmental Fate and Effects Division, pesticide environmental fate one line summary: DDT (p, p’) US Environmental Protection Agency Washington, DC Properties Review of Environmental Contamination and Toxicology. 1989:137.
-
- Eroschenko VP, Rourke AW, Sims WF. Estradiol or methoxychlor stimulates estrogen receptor (ER) expression in uteri. Reprod Toxicol. 1996;10(4):265–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

